Modulation of α(2C) adrenergic receptor temperature-sensitive trafficking by HSP90
- PMID: 21145921
- PMCID: PMC3148834
- DOI: 10.1016/j.bbamcr.2010.11.020
Modulation of α(2C) adrenergic receptor temperature-sensitive trafficking by HSP90
Abstract
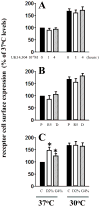
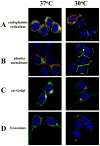
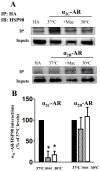
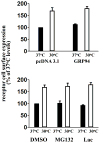
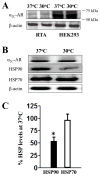
Decreasing the temperature to 30°C is accompanied by significant enhancement of α(2C)-AR plasma membrane levels in several cell lines with fibroblast phenotype, as demonstrated by radioligand binding in intact cells. No changes were observed on the effects of low-temperature after blocking receptor internalization in α(2C)-AR transfected HEK293T cells. In contrast, two pharmacological chaperones, dimethyl sulfoxide and glycerol, increased the cell surface receptor levels at 37°C, but not at 30°C. Further, at 37°C α(2C)-AR is co-localized with endoplasmic reticulum markers, but not with the lysosomal markers. Treatment with three distinct HSP90 inhibitors, radicicol, macbecin and 17-DMAG significantly enhanced α(2C)-AR cell surface levels at 37°C, but these inhibitors had no effect at 30°C. Similar results were obtained after decreasing the HSP90 cellular levels using specific siRNA. Co-immunoprecipitation experiments demonstrated that α(2C)-AR interacts with HSP90 and this interaction is decreased at 30°C. The contractile response to endogenous α(2C)-AR stimulation in rat tail artery was also enhanced at reduced temperature. Similar to HEK293T cells, HSP90 inhibition increased the α(2C)-AR contractile effects only at 37°C. Moreover, exposure to low-temperature of vascular smooth muscle cells from rat tail artery decreased the cellular levels of HSP90, but did not change HSP70 levels. These data demonstrate that exposure to low-temperature augments the α(2C)-AR transport to the plasma membrane by releasing the inhibitory activity of HSP90 on the receptor traffic, findings which may have clinical relevance for the diagnostic and treatment of Raynaud Phenomenon.
2010 Elsevier B.V. All rights reserved.
Figures






References
-
- Small KM, McGraw DW, Liggett SB. Pharmacology and physiology of human adrenergic receptor polymorphisms. Annu Rev Pharmacol Toxicol. 2003;43:381–411. - PubMed
-
- Saunders C, Limbird LE. Localization and trafficking of alpha2-adrenergic receptor subtypes in cells and tissues. Pharmacol Ther. 1999;84:193–205. - PubMed
-
- MacDonald E, Kobilka BK, Scheinin M. Gene targeting--homing in on alpha 2-adrenoceptor subtype function. Trends Pharmacol Sci. 1997;18:211–219. - PubMed
-
- Brede M, Philipp M, Knaus A, Muthig V, Hein L. Alpha2-adrenergic receptor subtypes – novel functions uncovered in gene-targeted mouse models. Biol Cell. 2004;96:343–348. - PubMed
-
- Knaus AE, Muthig V, Schickinger S, Moura E, Beetz N, Gilsbach R, Hein L. Alpha2-adrenoceptor subtypes--unexpected functions for receptors and ligands derived from gene-targeted mouse models. Neurochem Int. 2007;51:277–81. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

