Signaling cell death from the endoplasmic reticulum stress response
- PMID: 21146390
- PMCID: PMC3078187
- DOI: 10.1016/j.ceb.2010.11.003
Signaling cell death from the endoplasmic reticulum stress response
Abstract
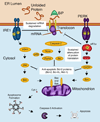
Inability to meet protein folding demands within the endoplasmic reticulum (ER) activates the unfolded protein response (UPR), a signaling pathway with both adaptive and apoptotic outputs. While some secretory cell types have a remarkable ability to increase protein folding capacity, their upper limits can be reached when pathological conditions overwhelm the fidelity and/or output of the secretory pathway. Irremediable 'ER stress' induces apoptosis and contributes to cell loss in several common human diseases, including type 2 diabetes and neurodegeneration. Researchers have begun to elucidate the molecular switches that determine when ER stress is too great to repair and the signals that are then sent from the UPR to execute the cell.
Copyright © 2010 Elsevier Ltd. All rights reserved.
Figures
References
-
-
Oyadomari S, Koizumi A, Takeda K, Gotoh T, Akira S, Araki E, Mori M. Targeted disruption of the Chop gene delays endoplasmic reticulum stress-mediated diabetes. J Clin Invest. 2002;109:525–532. ••Report that Chop signals ER stress-induced apoptosis in a diabetes model.
-
-
- Ma Y, Hendershot LM. ER chaperone functions during normal and stress conditions. J Chem Neuroanat. 2004;28:51–65. - PubMed
-
- Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007;8:519–529. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

