2,4-Diamino-5-(2'-arylpropargyl)pyrimidine derivatives as new nonclassical antifolates for human dihydrofolate reductase inhibition
- PMID: 21146434
- PMCID: PMC3073677
- DOI: 10.1016/j.jmgm.2010.11.004
2,4-Diamino-5-(2'-arylpropargyl)pyrimidine derivatives as new nonclassical antifolates for human dihydrofolate reductase inhibition
Abstract
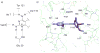
Dihydrofolate reductase (DHFR) has been a well-recognized target for the development of therapeutics for human cancers for several decades. Classical inhibitors of DHFR use an active transport mechanism to gain access to the cell; disabling this mechanism creates a pathway for resistance. In response, recent research focuses on nonclassical lipid-soluble DHFR inhibitors that are designed to passively diffuse through the membrane. Here, a new series of propargyl-linked antifolates are investigated as potential nonclassical human DHFR inhibitors. Several of these compounds exhibit potent enzyme inhibition with 50% inhibition concentration values under 500 nM. Molecular docking investigations show that the compounds maintain conserved hydrogen bonds between the pyrimidine ring and the enzyme as well as form van der Waals interactions with critical residues in the active site. Interestingly, the most potent compound, 2,4-diamino-5-(3-(3,4,5-trimethoxyphenyl)prop-1-ynyl)-6-ethylpyrimidine (compound 35), is 3500-fold more potent than trimethoprim, a potent inhibitor of bacterial DHFR but weak inhibitor of human DHFR. The two structural differences between compound 35 and trimethoprim show that the propargyl linkage and the substitution at C6 of the pyrimidine ring are critical to the formation of contacts with Thr 56, Ser 59, Ile 60, Leu 22, Phe 31 and Phe 34 and hence, to enhancing potency. The propargyl-linked antifolates are efficient ligands with a high ratio of potency to the number of non-hydrogen atoms and represent a potentially fruitful avenue for future development of antineoplastic agents.
Copyright © 2010 Elsevier Inc. All rights reserved.
Figures
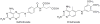


References
-
- Cody V, Galitsky N, Luft J, Pangborn W, Blakley R, Gangjee A. Comparison of ternary crystal complexes of F31 variants of human dihydrofolate reductase with NADPH and a classical antitumor furopyrimidine. Anti-Cancer Drug Des. 1998;13:307–15. - PubMed
-
- Cody V, Galitsky N, Luft J, Pangborn W, Rosowsky A, Blakley R. Comparison of two independent crystal structures of human dihydrofolate reductase ternary complexes reduced with nicotinamide adenine dinucleotide phosphate and the very tight-binding inhibitor PT523. Biochemistry. 1997;36:13897–903. - PubMed
-
- Lewis W, Cody V, Galitsky N, Luft J, Pangborn W, Chunduru S, Spencer HT, Appleman J, Blakley R. Methotrexate-resistant variants of human dihydrofolate reductase with substitutions of leucine 22. J Biol Chem. 1995;270:5057–64. - PubMed
-
- Rosowsky A, Bader H, Wright J, Keyomarsi K, Matherly L. Synthesis and biological activity of N-Hemiphthaloyl-α,ω-diaminoalkanoic acid analogues of aminopterin and 3′,5-dichloroaminopterin. J Med Chem. 1994;37:2167–74. - PubMed
-
- Gangjee A, Li W, Kisliuk R, Cody V, Pace J, Piraino J, Makin J. Design, synthesis and X-ray crystal structure of classical and nonclassical 2-amino-4-oxo-5-substituted-6-ethylthieno[2,3-d]pyrimidines as dual thymidylate synthase and dihdyrofolate reuctase inhibitors and as potential antitumor agents. J Med Chem. 2009;52:4892–902. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

