Oral priming with Salmonella Typhi vaccine strain CVD 909 followed by parenteral boost with the S. Typhi Vi capsular polysaccharide vaccine induces CD27+IgD-S. Typhi-specific IgA and IgG B memory cells in humans
- PMID: 21146460
- PMCID: PMC3035995
- DOI: 10.1016/j.clim.2010.11.006
Oral priming with Salmonella Typhi vaccine strain CVD 909 followed by parenteral boost with the S. Typhi Vi capsular polysaccharide vaccine induces CD27+IgD-S. Typhi-specific IgA and IgG B memory cells in humans
Abstract
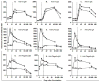
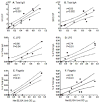
Attenuated live oral typhoid vaccine candidate CVD 909 constitutively expresses Salmonella Typhi capsular polysaccharide antigen (Vi). A randomized, double-blind, heterologous prime-boost clinical study was conducted to determine whether immunity to licensed parenteral Vi vaccine could be enhanced by priming with CVD 909. Priming with CVD 909 elicited higher and persistent, albeit not significant, anti-Vi IgG and IgA following immunization with Vi, than placebo-primed recipients. Vi-specific IgA B memory (B(M)) cells were significantly increased in CVD 909-primed subjects. S. Typhi-specific LPS and flagella IgA B(M) cells were observed in subjects immunized with CVD 909 or with the licensed Vi-negative oral typhoid vaccine Ty21a. CVD 909-induced B(M) cells exhibited a classical B(M) phenotype (i.e., CD3(-)CD19(+)IgD(-)CD27(+)). This is the first demonstration of classical B(M) cells specific for bacterial polysaccharide or protein antigens following typhoid immunization. The persistent IgA B(M) responses demonstrate the capacity of oral typhoid vaccines to prime mucosally relevant immune memory.
Copyright © 2010 Elsevier Inc. All rights reserved.
Conflict of interest statement
The remaining authors declare no conflicting financial interests.
Figures




References
-
- Klugman KP, Koornhof HJ, Robbins JB, Le Cam NN. Immunogenicity, efficacy and serological correlate of protection of Salmonella typhi Vi capsular polysaccharide vaccine three years after immunization. Vaccine. 1996;14:435–438. - PubMed
-
- Levine MM, Ferreccio C, Abrego P, Martin OS, Ortiz E, Cryz S. Duration of efficacy of Ty21a, attenuated Salmonella typhi live oral vaccine. Vaccine. 1999;17(Suppl 2):S22–S27. - PubMed
-
- Hessel L, Debois H, Fletcher M, Dumas R. Experience with Salmonella typhi Vi capsular polysaccharide vaccine. Eur J Clin Microbiol Infect Dis. 1999;18:609–620. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

