C. elegans Dicer interacts with the P-granule component GLH-1 and both regulate germline RNPs
- PMID: 21146518
- PMCID: PMC3031674
- DOI: 10.1016/j.ydbio.2010.12.005
C. elegans Dicer interacts with the P-granule component GLH-1 and both regulate germline RNPs
Abstract
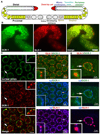
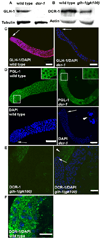
P granules, ribonucleoprotein (RNP) complexes specific to the cytoplasmic side of the nuclear pores of Caenorhabditis elegans germ cells, are implicated in post-transcriptional control of maternally-transcribed mRNAs. Here we show a relationship in C. elegans of Dicer, the riboendonuclease processing enzyme of the RNA interference and microRNA pathways, with GLH-1, a germline-specific RNA helicase and a constitutive component of P granules. Based on results from GST-pull-downs and immunoprecipitations, GLH-1 binds DCR-1 and this binding does not require RNA. Both GLH-1 protein and glh-1 mRNA levels are reduced in the dcr-1(ok247) null mutant background; conversely, a reduction of DCR-1 protein is observed in the glh-1(gk100) deletion strain. Thus, in the C. elegans germline, DCR-1 and GLH-1 are interdependent. In addition, evidence indicates that DCR-1 protein levels, like those of GLH-1, are likely regulated by the Jun N-terminal kinase (JNK), KGB-1. In adult germ cells, DCR-1 is found in uniformly-distributed, small puncta both throughout the cytoplasm and the nucleus, on the inner side of nuclear pores, and associated with P granules. In arrested oocytes, GLH-1 and DCR-1 re-localize to cytoplasmic and cortically-distributed RNP granules and are necessary to recruit other components to these complexes. We predict that the GLH-1/DCR-1 complex may function in the transport, deposition, or regulation of maternally-transcribed mRNAs and their associated miRNAs.
Copyright © 2010 Elsevier Inc. All rights reserved.
Figures






References
-
- Adler J, Parmryd I. Quantifying co-localization by correlation: the Pearson correlation coefficient is superior to the Mander's overlap coefficient. Cytometry Part A. 2010;77A:733–742. - PubMed
-
- Barbee SA, Lubin AL, Evans TC. A novel function for the Sm proteins in germ granule localization during C. elegans embryogenesis. Curr. Biol. 2002;12:1502–1506. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

