Elevated copper remodels hepatic RNA processing machinery in the mouse model of Wilson's disease
- PMID: 21146535
- PMCID: PMC3033566
- DOI: 10.1016/j.jmb.2010.12.001
Elevated copper remodels hepatic RNA processing machinery in the mouse model of Wilson's disease
Abstract
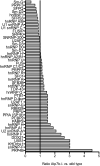
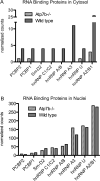
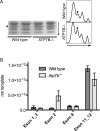
Copper is essential to mammalian physiology, and its homeostasis is tightly regulated. In humans, genetic defects in copper excretion result in copper overload and Wilson's disease (WD). Previous studies on the mouse model for WD (Atp7b(-)(/-)) revealed copper accumulation in hepatic nuclei and specific changes in mRNA profile prior to the onset of pathology. To find a molecular link between nuclear copper elevation and changes in hepatic transcriptome, we utilized quantitative ionomic and proteomic approaches. X-ray fluorescence and inductively coupled plasma mass spectrometry analysis indicate that copper in the Atp7b(-/-) nucleus, while highly elevated, does not markedly alter nuclear ion content. Widespread protein oxidation is also not observed, although the glutathione reductase SelH is upregulated, likely to maintain redox balance. We further demonstrate that accumulating copper affects the abundance and/or modification of a distinct subset of nuclear proteins. These proteins populate pathways that are most significantly associated with RNA processing. An alteration in splicing pattern was observed for hnRNP A2/B1, itself the RNA shuttling factor and spliceosome component. Analysis of hnRNP A2/B1 mRNA and protein revealed an increased retention of exon 2 and a selective 2-fold upregulation of a corresponding protein splice variant. Mass spectrometry measurements suggest that the nucleocytoplasmic distribution of RNA binding proteins, including hnRNP A2/B1, is altered in the Atp7b(-/-) liver. We conclude that remodeling of the RNA processing machinery is an important component of cell response to elevated copper that may guide pathology development in the early stages of WD.
Copyright © 2010 Elsevier Ltd. All rights reserved.
Figures




References
-
- Buiakova OI, Xu J, Lutsenko S, Zeitlin S, Das K, Das S, Ross BM, Mekios C, Scheinberg IH, Gilliam TC. Null mutation of the murine ATP7B (Wilson disease) gene results in intracellular copper accumulation and late-onset hepatic nodular transformation. Hum. Mol. Genet. 1999;8:1665–1671. - PubMed
-
- Harris ZL, Gitlin JD. Genetic and molecular basis for copper toxicity. Am J Clin Nutr. 1996;63:836S–841S. - PubMed
-
- Waggoner DJ, Bartnikas TB, Gitlin JD. The role of copper in neurodegenerative disease. Neurobiol Dis. 1999;6:221–230. - PubMed
-
- Carmichael PL, Hewer A, Osborne MR, Strain AJ, Phillips DH. Detection of bulky DNA lesions in the liver of patients with Wilson's disease and primary haemochromatosis. Mutat Res. 1995;326:235–243. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

