Increased lipogenesis, induced by AKT-mTORC1-RPS6 signaling, promotes development of human hepatocellular carcinoma
- PMID: 21147110
- PMCID: PMC3057329
- DOI: 10.1053/j.gastro.2010.12.006
Increased lipogenesis, induced by AKT-mTORC1-RPS6 signaling, promotes development of human hepatocellular carcinoma
Abstract
Background & aims: De novo lipogenesis is believed to be involved in oncogenesis. We investigated the role of aberrant lipid biosynthesis in the pathogenesis of human hepatocellular carcinoma (HCC).
Methods: We evaluated expression of enzymes that regulate lipogenesis in human normal liver tissues and HCC and surrounding, nontumor, liver tissues from patients using real-time reverse transcription polymerase chain reaction, immunoblotting, immunohistochemistry, and biochemical assays. Effects of lipogenic enzymes on human HCC cell lines were evaluated using inhibitors and overexpression experiments. The lipogenic role of the proto-oncogene AKT was assessed in vitro and in vivo.
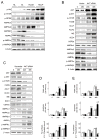
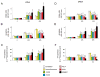
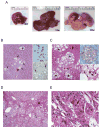
Results: In human liver samples, de novo lipogenesis was progressively induced from nontumorous liver tissue toward the HCC. Extent of aberrant lipogenesis correlated with clinical aggressiveness, activation of the AKT-mammalian target of rapamycin signaling pathway, and suppression of adenosine monophosphate-activated protein kinases. In HCC cell lines, the AKT-mammalian target of rapamycin complex 1-ribosomal protein S6 pathway promoted lipogenesis via transcriptional and post-transcriptional mechanisms that included inhibition of fatty acid synthase ubiquitination by the USP2a de-ubiquitinase and disruption of the SREBP1 and SREBP2 degradation complexes. Suppression of the genes adenosine triphosphate citrate lyase, acetyl-CoA carboxylase, fatty acid synthase, stearoyl-CoA desaturase 1, or sterol regulatory element-binding protein 1, which are involved in lipogenesis, reduced proliferation, and survival of HCC cell lines and AKT-dependent cell proliferation. Overexpression of an activated form of AKT in livers of mice induced lipogenesis and tumor development.
Conclusions: De novo lipogenesis has pathogenic and prognostic significance for HCC. Inhibitors of lipogenic signaling, including those that inhibit the AKT pathway, might be useful as therapeutics for patients with liver cancer.
Copyright © 2011 AGA Institute. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures




References
-
- Bruix J, Boix L, Sala M, et al. Focus on hepatocellular carcinoma. Cancer Cell. 2004;5:215–219. - PubMed
-
- Thorgeirsson SS, Grisham JW. Molecular pathogenesis of human hepatocellular carcinoma. Nat Genet. 2002;31:339–346. - PubMed
-
- Hajer GR, van Haeften TW, Visseren FL. Adipose tissue dysfunction in obesity, diabetes, and vascular diseases. Eur Heart J. 2008;29:2959–2971. - PubMed
-
- Kuhajda FP. Fatty-acid Synthase and human cancer: new perspectives on its role in tumor biology. Nutrition. 2000;16:202–208. - PubMed
-
- Swinnen JV, Brusselmans K, Verhoeven G. Increased lipogenesis in cancer cells: new players, novel targets. Curr Opin Clin Nutr Metab Care. 2006;9:358–365. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

