Mitochondrial protein import and the genesis of steroidogenic mitochondria
- PMID: 21147195
- PMCID: PMC3057322
- DOI: 10.1016/j.mce.2010.12.007
Mitochondrial protein import and the genesis of steroidogenic mitochondria
Abstract
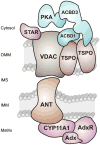
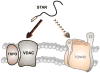
The principal site of regulation of steroid hormone biosynthesis is the transfer of cholesterol from the outer to inner mitochondrial membrane. Hormonal stimulation of steroidogenic cells promotes this mitochondrial lipid import through a multi-protein complex, termed the transduceosome, spanning the two membranes. The transduceosome complex is assembled from multiple proteins, such as the steroidogenic acute regulatory (STAR) protein and translocator protein (TSPO), and requires their targeting to the mitochondria for transduceosome function. The vast majority of mitochondrial proteins, including those participating in cholesterol import, are encoded in the nucleus. Their subsequent mitochondrial incorporation is performed through a series of protein import machineries located in the outer and inner mitochondrial membranes. Here we review our current knowledge of the mitochondrial cholesterol import machinery of the transduceosome. This is complemented with descriptions of mitochondrial protein import machineries and mechanisms by which these machineries assemble the transduceosome in steroidogenic mitochondria.
Copyright © 2010 Elsevier Ireland Ltd. All rights reserved.
Figures


References
-
- Abe Y, Shodai T, Muto T, Mihara K, Torii H, Nishikawa S, Endo T, Kohda D. Structural basis of presequence recognition by the mitochondrial protein import receptor Tom20. Cell. 2000;100:551–560. - PubMed
-
- Ahting U, Waizenegger T, Neupert W, Rapaport D. Signal-anchored proteins follow a unique insertion pathway into the outer membrane of mitochondria. J Biol Chem. 2005;280:48–53. - PubMed
-
- Arlt H, Tauer R, Feldmann H, Neupert W, Langer T. The YTA10-12 complex, an AAA protease with chaperone-like activity in the inner membrane of mitochondria. Cell. 1996;85:875–885. - PubMed
-
- Armstrong LC, Komiya T, Bergman BE, Mihara K, Bornstein P. Metaxin is a component of a preprotein import complex in the outer membrane of the mammalian mitochondrion. J Biol Chem. 1997;272:6510–6518. - PubMed
-
- Armstrong LC, Saenz AJ, Bornstein P. Metaxin 1 interacts with metaxin 2, a novel related protein associated with the mammalian mitochondrial outer membrane. J Cell Biochem. 1999;74:11–22. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

