Differential requirement for Caspase-1 autoproteolysis in pathogen-induced cell death and cytokine processing
- PMID: 21147462
- PMCID: PMC3016200
- DOI: 10.1016/j.chom.2010.11.007
Differential requirement for Caspase-1 autoproteolysis in pathogen-induced cell death and cytokine processing
Abstract
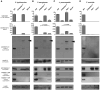
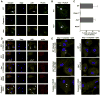
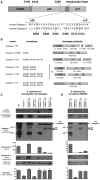
Activation of the cysteine protease Caspase-1 is a key event in the innate immune response to infections. Synthesized as a proprotein, Caspase-1 undergoes autoproteolysis within multiprotein complexes called inflammasomes. Activated Caspase-1 is required for proteolytic processing and for release of the cytokines interleukin-1β and interleukin-18, and it can also cause rapid macrophage cell death. We show that macrophage cell death and cytokine maturation in response to infection with diverse bacterial pathogens can be separated genetically and that two distinct inflammasome complexes mediate these events. Inflammasomes containing the signaling adaptor Asc form a single large "focus" in which Caspase-1 undergoes autoproteolysis and processes IL-1β/IL-18. In contrast, Asc-independent inflammasomes activate Caspase-1 without autoproteolysis and do not form any large structures in the cytosol. Caspase-1 mutants unable to undergo autoproteolysis promoted rapid cell death, but processed IL-1β/18 inefficiently. Our results suggest the formation of spatially and functionally distinct inflammasomes complexes in response to bacterial pathogens.
Copyright © 2010 Elsevier Inc. All rights reserved.
Figures
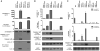


References
-
- Amer A, Franchi L, Kanneganti TD, Body-Malapel M, Ozoren N, Brady G, Meshinchi S, Jagirdar R, Gewirtz A, Akira S, Nunez G. Regulation of Legionella phagosome maturation and infection through flagellin and host Ipaf. J Biol Chem. 2006;281:35217–35223. - PubMed
-
- Bao Q, Shi Y. Apoptosome: a platform for the activation of initiator caspases. Cell Death Differ. 2007;14:56–65. - PubMed
-
- Blasi E, Mathieson BJ, Varesio L, Cleveland JL, Borchert PA, Rapp UR. Selective immortalization of murine macrophages from fresh bone marrow by a raf/myc recombinant murine retrovirus. Nature. 1985;318:667–670. - PubMed
-
- Boatright KM, Renatus M, Scott FL, Sperandio S, Shin H, Pedersen IM, Ricci JE, Edris WA, Sutherlin DP, Green DR, Salvesen GS. A unified model for apical caspase activation. Mol Cell. 2003;11:529–541. - PubMed
-
- Boyden ED, Dietrich WF. Nalp1b controls mouse macrophage susceptibility to anthrax lethal toxin. Nat Genet. 2006;38:240–244. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

