Role of the EASL, RECIST, and WHO response guidelines alone or in combination for hepatocellular carcinoma: radiologic-pathologic correlation
- PMID: 21147504
- PMCID: PMC3094725
- DOI: 10.1016/j.jhep.2010.10.004
Role of the EASL, RECIST, and WHO response guidelines alone or in combination for hepatocellular carcinoma: radiologic-pathologic correlation
Abstract
Background & aims: We sought to study receiver-operating characteristics (ROC) of the European Association for the Study of the Liver (EASL), Response Evaluation Criteria in Solid Tumors (RECIST), and World Health Organization (WHO) guidelines for assessing response following locoregional therapies individually and in various combinations.
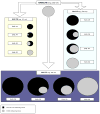
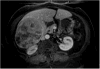
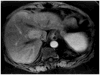
Methods: Eighty-one patients with hepatocellular carcinoma underwent liver explantation following locoregional therapies. Response was assessed using EASL, RECIST, and WHO. Kappa statistics were used to determine inter-method agreement. Uni/multivariate logistic regression analyses were performed to determine the variables predicting complete pathologic necrosis. Numerical values were assigned to the response classes: complete response=0, partial response=1, stable disease=2, and progressive disease=3. Various mathematical combinations of EASL and WHO were tested to calculate scores and their ROCs were studied using pathological examination of the explant as the gold standard.
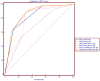
Results: Median times (95% CI) to the WHO, RECIST, and EASL responses were 5.3 (4-11.5), 5.6 (4-11.5), and 1.3months (1.2-1.5), respectively. Kappa coefficients for WHO/RECIST, WHO/EASL, and RECIST/EASL were 0.78, 0.28, and 0.31, respectively. EASL response demonstrated significant odds ratios for predicting complete pathologic necrosis on uni/multivariate analyses. Calculated areas under the ROC curves were: RECIST: 0.63, WHO: 0.68, EASL: 0.82, EASL+WHO: 0.82, EASL×WHO: 0.85, EASL+(2×WHO): 0.79 and (2×EASL)+WHO: 0.85. An EASL×WHO Score of ⩽1 had 90.2% sensitivity for predicting complete pathologic necrosis.
Conclusions: The product of WHO and EASL demonstrated better ROC than the individual guidelines for assessment of tumor response. EASL×WHO scoring system provides a simple and clinically applicable method of response assessment following locoregional therapies for hepatocellular carcinoma.
Copyright © 2010 European Association for the Study of the Liver. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Figures



References
-
- El-Serag HB. Hepatocellular carcinoma and hepatitis C in the United States. Hepatology. 2002;36(5 Suppl 1):S74–83. - PubMed
-
- Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334(11):693–699. - PubMed
-
- Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359(4):378–390. - PubMed
-
- Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10(1):25–34. - PubMed
-
- Salem R, Lewandowski RJ, Mulcahy MF, Riaz A, Ryu RK, Ibrahim S, et al. Radioembolization for hepatocellular carcinoma using Yttrium-90 microspheres: a comprehensive report of long-term outcomes. Gastroenterology. 2010;138(1):52–64. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

