Fatty acid (FFA) transport in cardiomyocytes revealed by imaging unbound FFA is mediated by an FFA pump modulated by the CD36 protein
- PMID: 21147770
- PMCID: PMC3039365
- DOI: 10.1074/jbc.M110.182162
Fatty acid (FFA) transport in cardiomyocytes revealed by imaging unbound FFA is mediated by an FFA pump modulated by the CD36 protein
Abstract
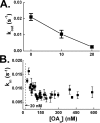
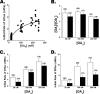
Free fatty acid (FFA) transport across the cardiomyocyte plasma membrane is essential to proper cardiac function, but the role of membrane proteins and FFA metabolism in FFA transport remains unclear. Metabolism is thought to maintain intracellular FFA at low levels, providing the driving force for FFA transport, but intracellular FFA levels have not been measured directly. We report the first measurements of the intracellular unbound FFA concentrations (FFA(i)) in cardiomyocytes. The fluorescent indicator of FFA, ADIFAB (acrylodan-labeled rat intestinal fatty acid-binding protein), was microinjected into isolated cardiomyocytes from wild type (WT) and FAT/CD36 null C57B1/6 mice. Quantitative imaging of ADIFAB fluorescence revealed the time courses of FFA influx and efflux. For WT mice, rate constants for efflux (∼0.02 s(-1)) were twice influx, and steady state FFA(i) were more than 3-fold larger than extracellular unbound FFA (FFA(o)). The concentration gradient and the initial rate of FFA influx saturated with increasing FFA(o). Similar characteristics were observed for oleate, palmitate, and arachidonate. FAT/CD36 null cells revealed similar characteristics, except that efflux was 2-3-fold slower than WT cells. Rate constants determined with intracellular ADIFAB were confirmed by measurements of intracellular pH. FFA uptake by suspensions of cardiomyocytes determined by monitoring FFA(o) using extracellular ADIFAB confirmed the influx rate constants determined from FFA(i) measurements and demonstrated that rates of FFA transport and etomoxir-sensitive metabolism are regulated independently. We conclude that FFA influx in cardiac myocytes is mediated by a membrane pump whose transport rate constants may be modulated by FAT/CD36.
Figures
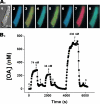
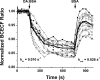
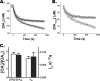

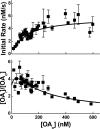
References
-
- Szczepaniak L. S., Victor R. G., Orci L., Unger R. H. (2007) Circ. Res. 101, 759–767 - PubMed
-
- Kampf J. P., Kleinfeld A. M. (2007) Physiology 22, 7–14 - PubMed
-
- Bonen A., Chabowski A., Luiken J. J., Glatz J. F. (2007) Physiology 22, 15–29 - PubMed
-
- Hamilton J. A. (2007) Prostaglandins Leukot. Essent. Fatty Acids 77, 355–361 - PubMed
-
- Ibrahimi A., Abumrad N. A. (2002) Curr. Opin. Clin. Nutr. Metab Care 5, 139–145 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

