The interaction between tropomyosin-related kinase B receptors and presynaptic muscarinic receptors modulates transmitter release in adult rodent motor nerve terminals
- PMID: 21147991
- PMCID: PMC6634863
- DOI: 10.1523/JNEUROSCI.2676-10.2010
The interaction between tropomyosin-related kinase B receptors and presynaptic muscarinic receptors modulates transmitter release in adult rodent motor nerve terminals
Abstract
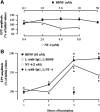
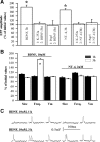
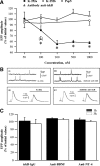
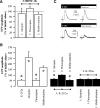
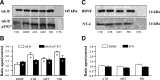
The neurotrophin brain-derived neurotrophic factor (BDNF), neurotrophin-4 (NT-4) and the receptors tropomyosin-related kinase B (trkB) and p75(NTR) are present in the nerve terminals on the neuromuscular junctions (NMJs) of the levator auris longus muscle of the adult mouse. Exogenously added BDNF or NT-4 increased evoked ACh release after 3 h. This presynaptic effect (the size of the spontaneous potentials is not affected) is specific because it is not produced by neurotrophin-3 (NT-3) and is prevented by preincubation with trkB-IgG chimera or by pharmacological block of trkB [K-252a (C₂₇H₂₁N₃O₅)] or p75(NTR) [Pep5 (C₈₆H₁₁₁N₂₅O₁₉S₂] signaling. The effect of BDNF depends on the M₁ and M₂ muscarinic acetylcholine autoreceptors (mAChRs) because it is prevented by atropine, pirenzepine and methoctramine. We found that K-252a incubation reduces ACh release (~50%) in a short time (1 h), but the p75(NTR) signaling inhibitor Pep5 does not have this effect. The specificity of the K-252a blocking effect on trkB was confirmed with the anti-trkB antibody 47/trkB, which reduces evoked ACh release, like K-252a, whereas the nonpermeant tyrosine kinase blocker K-252b does not. Neither does incubation with the fusion protein trkB-IgG (to chelate endogenous BDNF/NT-4), anti-BDNF or anti-NT-4 change ACh release. Thus, the trkB receptor normally seems to be coupled to ACh release when there is no short-term local effect of neurotrophins at the NMJ. The normal function of the mAChR mechanism is a permissive prerequisite for the trkB pathway to couple to ACh release. Reciprocally, the normal function of trkB modulates M₁- and M₂-subtype muscarinic pathways.
Figures

References
-
- Alsina B, Vu T, Cohen-Cory S. Visualizing synapse formation in arborizing optic axons in vivo: dynamics and modulation by BDNF. Nat Neurosci. 2001;4:1093–1101. - PubMed
-
- Barbacid M. The Trk family of neurotrophin receptors. J Neurobiol. 1994;25:1386–1403. - PubMed
-
- Bibel M, Barde YA. Neurotrophins: key regulators of cell fate and cell shape in the vertebrate nervous system. Genes Dev. 2000;14:2919–2937. - PubMed
-
- Boulanger LM, Poo MM. Presynaptic depolarization facilitates neurotrophin-induced synaptic potentiation. Nat Neurosci. 1999;2:346–351. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
