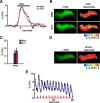
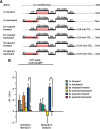
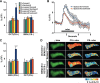
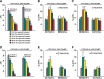
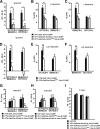
Figure 4.
The memory trace in the axons of γ MB neurons forms between 9 and 18 h and persists until 48 h after spaced forward conditioning. A, Time course for long-term memory of flies carrying 1471–Gal4 and Uas–G-CaMP. Memory after spaced forward conditioning decreases with time. Flies were trained using the 5× spaced forward conditioning protocol with Oct (CS+) versus Ben (CS−) and Ben (CS+) versus Oct (CS−) and tested 3, 9, 18, 24, 48, and 96 h later for behavioral memory (ΔPI). For Oct (CS+), the performance of the flies gradually decreased with time (Kruskal–Wallis statistic of 38.215, p < 0.0001), and flies tested at 3 and 9 h performed significantly better than flies tested at 24, 48, and 96 h (Mann–Whitney pairwise comparisons, p ≤ 0.0047). Flies tested at 18 h demonstrated significantly higher performance scores compared with flies tested at 48 and 96 h (Mann–Whitney pairwise comparisons, p ≤ 0.0179). For Ben (CS+), flies tested at 3 h performed significantly better than all other time points (Kruskal–Wallis statistic of 26.776, p < 0.0001; Mann–Whitney pairwise comparisons, p ≤ 0.0024). The PI scores for flies tested at the other time points were not significantly different from each other (Mann–Whitney pairwise comparisons, p ≥ 0.0865), except that the PI score for flies tested at 96 h was significantly different from 9 and 24 h (Mann–Whitney pairwise comparisons, p ≤ 0.0084; n = 10–12 for all groups). Error bars are the SEM. B, Time course for the cellular memory trace in the axons of the γ MB neurons with Oct (CS+). There was a significant increase in the measured calcium transients (%ΔF/F0) in response to the CS+ (Oct) at 18, 24, and 48 h after spaced forward conditioning compared with 3, 9, and 96 h (Kruskal–Wallis statistic of 22.504, p = 0.0004; Mann–Whitney pairwise comparisons, p ≤ 0.0233). There were no significant differences among the 18, 24, and 48 time points (Kruskal–Wallis statistic of 0.234, p = 0.8894; Mann–Whitney pairwise comparisons, p ≥ 0.0741) or the 3, 9, and 96 h time points (Kruskal–Wallis statistic of 0.611, p = 0.7368; Mann–Whitney pairwise comparisons, p ≥ 0.5078). There were no significant differences in response to the CS− (Ben) among any of the time points (Kruskal–Wallis statistic of 2.159, p = 0.8268; Mann–Whitney pairwise comparisons, p ≥ 0.0715; n = 8–20 for all groups). Error bars are the SEM. *p < 0.05, **p < 0.01. C, Time course for the cellular memory trace in the axons of the γ MB neurons with Ben (CS+). There was a significant increase in the measured calcium transients (%ΔF/F0) in response to the CS+ (Ben) in the axons of the γ MB neurons at 18, 24, and 48 h after spaced forward conditioning compared with 3, 9, and 96 h (Kruskal–Wallis statistic of 23.247, p = 0.0003; Mann–Whitney pairwise comparisons, p ≤ 0.0318) except for the response at 48 and 96 h (Mann–Whitney test, p = 0.0723). There were no significant differences among the18, 24, and 48 h time points (Kruskal–Wallis statistic of 1.394, p = 0.498; Mann–Whitney pairwise comparisons, p ≥ 0.3181) or the 3, 9, and 96 h time points (Kruskal–Wallis statistic of 0.479, p = 0.7870; Mann–Whitney pairwise comparisons, p ≥ 0.4328). There were no significant differences in response to the CS− (Ben) among any time point (Kruskal–Wallis statistic of 4.431, p = 0.4891; Mann–Whitney pairwise comparisons, p ≥ 0.1546; n = 8–15 for all groups). Error bars are the SEM. *p < 0.05, **p < 0.01. D, Time course of long-term memory for flies carrying c739–Gal4 and Uas–G-CaMP. Memory after spaced forward conditioning decreases with time. Flies were trained using the 5× spaced forward conditioning protocol with Oct (CS+) versus Ben (CS−) and Ben (CS+) versus Oct (CS−) and tested at different time points for behavioral memory (ΔPI). For Oct (CS+), the performance of the flies gradually decreased with time (Kruskal–Wallis statistic of 37.828, p < 0.0001), and all pairwise comparisons were significantly different from one another (Mann–Whitney test, p ≤ 0.0479) except for the 3 vs 9 h and 48 vs 96 h PI values (Mann–Whitney test, p ≥ 0.0647). Similarly, for Ben (CS+), the performance of the flies gradually decreased with time (Kruskal–Wallis statistic of 34.018, p < 0.0001), and all pairwise comparisons were significantly different from one another (Mann–Whitney test, p ≤ 0.0433) except for the performance at 9 h, which was not significantly different from 3 and 24 h (Mann–Whitney test, p ≥ 0.0865; n = 10–12 for all groups). Error bars are the SEM. E, Time course for the cellular memory trace in the α branch of the α/β MB neurons with Oct (CS+). There was significant increment in the measured calcium transients (%ΔF/F0) in response to Oct (CS+) at 9 and 24 h after spaced conditioning compared with 3 and 48 h (Kruskal–Wallis statistic of 16.312, p = 0.0010; Mann–Whitney pairwise comparisons, p ≤ 0.0138). There was no significant difference between the 9 and 24 h time points (Mann–Whitney pairwise comparisons, p = 0.3823) or the 3 and 48 h time points (Mann–Whitney pairwise comparisons, p = 0.902). In addition, there were no significant differences in response to the CS− among the different time points (Kruskal–Wallis statistic of 2.595, p = 0.4583; Mann–Whitney pairwise comparisons, p ≥ 0.0743). Data for 3, 9, and 24 h time points are reproduced from Yu et al. (2006). n = 9–12 for all groups. Error bars are the SEM. *p < 0.05, **p < 0.01. F, Time course for the cellular memory trace in the α branch of the α/β MB neurons with Ben (CS+). There was significant increase in the measured calcium transients (%ΔF/F0) in response to the CS+ (Ben) at 9 and 24 h after spaced conditioning training compared with 3 and 48 h (Kruskal–Wallis statistic of 14.378, p = 0.0024; Mann–Whitney pairwise comparisons, p ≤ 0.0128). There was no significant difference between the 9 and 24 h time point (Mann–Whitney test, p = 0.4469) or between the 3 and 48 h time points (Mann–Whitney test, p = 0.7913). In addition, there were no significant differences in response to the CS− among the different time points (Kruskal–Wallis statistic of 2.365, p = 0.5002; Mann–Whitney pairwise comparisons, p ≥ 0.1604). Data for the 24 h time point are reproduced from Yu et al. (2006). n = 7–12 for all groups. Error bars are the SEM. *p < 0.05, **p < 0.01.