Novel insights into the regulation of skeletal muscle protein synthesis as revealed by a new nonradioactive in vivo technique
- PMID: 21148113
- PMCID: PMC3042844
- DOI: 10.1096/fj.10-168799
Novel insights into the regulation of skeletal muscle protein synthesis as revealed by a new nonradioactive in vivo technique
Abstract
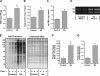
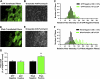
In this study, the principles of surface sensing of translation (SUnSET) were used to develop a nonradioactive method for ex vivo and in vivo measurements of protein synthesis (PS). Compared with controls, we first demonstrate excellent agreement between SUnSET and a [(3)H]phenylalanine method when detecting synergist ablation-induced increases in skeletal muscle PS ex vivo. We then show that SUnSET can detect the same synergist ablation-induced increase in PS when used in vivo (IV-SUnSET). In addition, IV-SUnSET detected food deprivation-induced decreases in PS in the heart, kidney, and skeletal muscles, with similar changes being visualized with an immunohistochemical version of IV-SUnSET (IV-IHC-SUnSET). By combining IV-IHC-SUnSET with in vivo transfection, we demonstrate that constitutively active PKB induces a robust increase in skeletal muscle PS. Furthermore, transfection with Ras homolog enriched in brain (Rheb) revealed that a PKB-independent activation of mammalian target of rapamycin is also sufficient to induce an increase in skeletal muscle PS. Finally, IV-IHC-SUnSET exposed the existence of fiber type-dependent differences in skeletal muscle PS, with PS in type 2B and 2X fibers being significantly lower than that in type 2A fibers within the same muscle. Thus, our nonradioactive method allowed us to accurately visualize and quantify PS under various ex vivo and in vivo conditions and revealed novel insights into the regulation of PS in skeletal muscle.
Figures
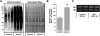
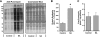



References
-
- Wolfe R. R., Chinkes D. L. (2005) Isotope Tracers in Metabolic Research, John Wiley & Sons, Hoboken, NJ, USA
-
- Schmidt E. K., Clavarino G., Ceppi M., Pierre P. (2009) SUnSET, a nonradioactive method to monitor protein synthesis. Nat. Methods 6, 275–277 - PubMed
-
- Sandri M. (2008) Signaling in muscle atrophy and hypertrophy. Physiology (Bethesda) 23, 160–170 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

