Syndecan-1 and syndecan-2 play key roles in herpes simplex virus type-1 infection
- PMID: 21148276
- PMCID: PMC3133699
- DOI: 10.1099/vir.0.027052-0
Syndecan-1 and syndecan-2 play key roles in herpes simplex virus type-1 infection
Abstract
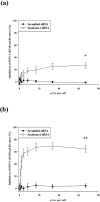
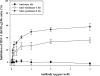
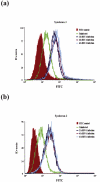
Herpes simplex virus type 1 (HSV-1) is an important human pathogen and a leading cause of infectious blindness in the developed world. HSV-1 exploits heparan sulfate proteoglycans (HSPG) for attachment to cells. While the significance of heparan sulphate (HS) moieties in HSV-1 infection is well established, the role of specific proteoglycan core proteins in the infection process remains poorly understood. The objective of this study was to assess the roles of syndecan-1 and syndecan-2 core proteins in HSV-1 infection, both of which are expressed by many HSV-1 target cell types. Our results demonstrate that syndecan-1 and syndecan-2 gene silencing by RNA interference reduces HSV-1 entry, plaque formation and facilitates cell survival. Furthermore, HSV-1 infection increases syndecan-1 and syndecan-2 protein synthesis and a resultant increase in cell surface expression of HS. Our observations suggest that changes in syndecan-1 and syndecan-2 expression levels may be related to active viral infection. Taken together, our findings provide new insights into HSPG functions during HSV-1 entry and spread.
Figures




References
-
- Anastasiadou, E., Vaeth, S., Cuomo, L., Boccellato, F., Vincenti, S., Cirone, M., Presutti, C., Junker, S., Winberg, G. & Frati, L. (2009). Epstein–Barr virus infection leads to partial phenotypic reversion of terminally differentiated malignant B cells. Cancer Lett 284, 165–174. - PubMed
-
- Barth, H., Schafer, C., Adah, M. I., Zhang, F., Linhardt, R. J., Toyoda, H., Kinoshita-Toyoda, A., Toida, T., Van Kuppevelt, T. H. & other authors (2003). Cellular binding of hepatitis C virus envelope glycoprotein E2 requires cell surface heparan sulfate. J Biol Chem 278, 41003–41012. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

