Actin cables and the exocyst form two independent morphogenesis pathways in the fission yeast
- PMID: 21148300
- PMCID: PMC3016976
- DOI: 10.1091/mbc.E10-08-0720
Actin cables and the exocyst form two independent morphogenesis pathways in the fission yeast
Abstract
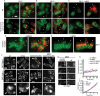
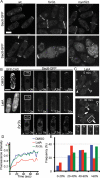
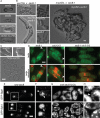
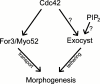
Cell morphogenesis depends on polarized exocytosis. One widely held model posits that long-range transport and exocyst-dependent tethering of exocytic vesicles at the plasma membrane sequentially drive this process. Here, we describe that disruption of either actin-based long-range transport and microtubules or the exocyst did not abolish polarized growth in rod-shaped fission yeast cells. However, disruption of both actin cables and exocyst led to isotropic growth. Exocytic vesicles localized to cell tips in single mutants but were dispersed in double mutants. In contrast, a marker for active Cdc42, a major polarity landmark, localized to discreet cortical sites even in double mutants. Localization and photobleaching studies show that the exocyst subunits Sec6 and Sec8 localize to cell tips largely independently of the actin cytoskeleton, but in a cdc42 and phospholipid phosphatidylinositol 4,5-bisphosphate (PIP₂)-dependent manner. Thus in fission yeast long-range cytoskeletal transport and PIP₂-dependent exocyst represent parallel morphogenetic modules downstream of Cdc42, raising the possibility of similar mechanisms in other cell types.
Figures


References
-
- Balasubramanian MK, Helfman DM, Hemmingsen SM. A new tropomyosin essential for cytokinesis in the fission yeast S. pombe. Nature. 1992;360:84–87. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

