Mast cells, macrophages, and crown-like structures distinguish subcutaneous from visceral fat in mice
- PMID: 21148461
- PMCID: PMC3035684
- DOI: 10.1194/jlr.M011338
Mast cells, macrophages, and crown-like structures distinguish subcutaneous from visceral fat in mice
Abstract
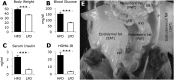
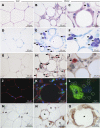
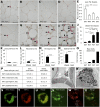
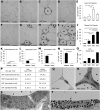
Obesity is accompanied by adipocyte death and accumulation of macrophages and mast cells in expanding adipose tissues. Considering the differences in biological behavior of fat found in different anatomical locations, we explored the distribution of mast cells, solitary macrophages, and crown-like structures (CLS), the surrogates for dead adipocytes, in subcutaneous and abdominal visceral fat of lean and diet-induced obese C57BL/6 mice. In fat depots of lean mice, mast cells were far less prevalent than solitary macrophages. Subcutaneous fat contained more mast cells, but fewer solitary macrophages and CLS, than visceral fat. Whereas no significant change in mast cell density of subcutaneous fat was observed, obesity was accompanied by a substantial increase in mast cells in visceral fat. CLS became prevalent in visceral fat of obese mice, and the distribution paralleled mast cells. Adipose tissue mast cells contained and released preformed TNF-α, the cytokine implicated in the pathogenesis of obesity-linked insulin resistance. In summary, subcutaneous fat differed from visceral fat by immune cell composition and a lower prevalence of CLS both in lean and obese mice. The increase in mast cells in visceral fat of obese mice suggests their role in the pathogenesis of obesity and insulin resistance.
Figures

References
-
- Mokdad A. H., Ford E. S., Bowman B. A., Dietz W. H., Vinicor F., Bales V. S., Marks J. S. 2003. Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. JAMA. 289: 76–79. - PubMed
-
- Hotamisligil G. S. 2006. Inflammation and metabolic disorders. Nature. 444: 860–867. - PubMed
-
- Hotamisligil G. S., Shargill N. S., Spiegelman B. M. 1993. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 259: 87–91. - PubMed
-
- Uysal K. T., Wiesbrock S. M., Marino M. W., Hotamisligil G. S. 1997. Protection from obesity-induced insulin resistance in mice lacking TNF-alpha function. Nature. 389: 610–614. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

