Potent CD8+ T-cell immunogenicity in humans of a novel heterosubtypic influenza A vaccine, MVA-NP+M1
- PMID: 21148512
- PMCID: PMC3060888
- DOI: 10.1093/cid/ciq015
Potent CD8+ T-cell immunogenicity in humans of a novel heterosubtypic influenza A vaccine, MVA-NP+M1
Abstract
Background: Influenza A viruses cause occasional pandemics and frequent epidemics. Licensed influenza vaccines that induce high antibody titers to the highly polymorphic viral surface antigen hemagglutinin must be re-formulated and readministered annually. A vaccine providing protective immunity to the highly conserved internal antigens could provide longer-lasting protection against multiple influenza subtypes.
Methods: We prepared a Modified Vaccinia virus Ankara (MVA) vector encoding nucleoprotein and matrix protein 1 (MVA-NP+M1) and conducted a phase I clinical trial in healthy adults.
Results: The vaccine was generally safe and well tolerated, with significantly fewer local side effects after intramuscular rather than intradermal administration. Systemic side effects increased at the higher dose in both frequency and severity, with 5 out of 8 volunteers experiencing severe nausea/vomiting, malaise, or rigors. Ex vivo T-cell responses to NP and M1 measured by IFN-γ ELISPOT assay were significantly increased after vaccination (prevaccination median of 123 spot-forming units/million peripheral blood mononuclear cells, postvaccination peak response median 339, 443, and 1443 in low-dose intradermal, low-dose intramuscular, and high-dose intramuscular groups, respectively), and the majority of the antigen-specific T cells were CD8(+).
Conclusions: We conclude that the vaccine was both safe and remarkably immunogenic, leading to frequencies of responding T cells that appear to be much higher than those induced by any other influenza vaccination approach. Further studies will be required to find the optimum dose and to assess whether the increased T-cell response to conserved influenza proteins results in protection from influenza disease.
Figures
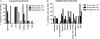

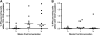

Comment in
-
Editorial commentary: a better grip: T cells strengthen our hand against influenza.Clin Infect Dis. 2011 Jan 1;52(1):8-9. doi: 10.1093/cid/ciq018. Clin Infect Dis. 2011. PMID: 21148513 No abstract available.
References
-
- Centers for Disease Control and Prevention (CDC) Interim within-season estimate of the effectiveness of trivalent inactivated influenza vaccine–Marshfield, Wisconsin, 2007-08 influenza season. MMWR Morb Mortal Wkly Rep. 2008;57:393–398. - PubMed
-
- de Jong JC, Beyer WE, Palache AM, Rimmelzwaan GF, Osterhaus AD. Mismatch between the 1997/1998 influenza vaccine and the major epidemic A(H3N2) virus strain as the cause of an inadequate vaccine-induced antibody response to this strain in the elderly. J Med Virol. 2000;61:94–99. - PubMed
-
- Nolan TM, Richmond PC, Skeljo MV, et al. Phase I and II randomised trials of the safety and immunogenicity of a prototype adjuvanted inactivated split-virus influenza A (H5N1) vaccine in healthy adults. Vaccine. 2008;26:4160–4167. - PubMed
-
- Breathnach CC, Clark HJ, Clark RC, Olsen CW, Townsend HG, Lunn DP. Immunization with recombinant modified vaccinia Ankara (rMVA) constructs encoding the HA or NP gene protects ponies from equine influenza virus challenge. Vaccine. 2006;24:1180–1190. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

