Peroxisome proliferator-activated receptor gamma negatively regulates IFN-beta production in Toll-like receptor (TLR) 3- and TLR4-stimulated macrophages by preventing interferon regulatory factor 3 binding to the IFN-beta promoter
- PMID: 21148557
- PMCID: PMC3037665
- DOI: 10.1074/jbc.M110.149823
Peroxisome proliferator-activated receptor gamma negatively regulates IFN-beta production in Toll-like receptor (TLR) 3- and TLR4-stimulated macrophages by preventing interferon regulatory factor 3 binding to the IFN-beta promoter
Abstract
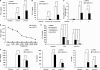
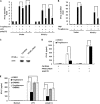
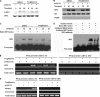
Toll-like receptors 3 and 4 utilize adaptor TRIF to activate interferon regulatory factor 3 (IRF3), resulting in IFN-β production to mediate anti-viral and bacterial infection. Peroxisome proliferator-activated receptor (PPAR)-γ is a ligand-activated transcription factor expressed in various immune cells and acts as a transcriptional repressor to inhibit the transcription of many proinflammatory cytokines. But, the function of PPAR-γ in TLR3- and -4-mediated IFN-β production is not well elucidated. Here, we have analyzed the effect of the PPAR-γ agonists on IFN-β production in peritoneal primary macrophages in response to LPS and poly(I:C). PPAR-γ agonists inhibited LPS and poly(I:C)-induced IFN-β transcription and secretion. siRNA knockdown of PPAR-γ expression and transfection of PPAR-γ expression plasmid demonstrated that PPAR-γ agonist inhibits IFN-β production in a PPAR-γ-dependent manner. The ability of the PPAR-γ agonist to inhibit IFN-β production was confirmed in vivo as mice treated with troglitazone exhibited decreased levels of IFN-β upon LPS and poly(I:C) challenge. Chromatin immunoprecipitation (CHIP) assay and electrophoretic mobility shift assay (EMSA) demonstrated that troglitazone treatment impaired IRF3 binding to the IFN-β promoter. Furthermore, troglitazone could inhibit LPS and poly(I:C)-induced STAT1 phosphorylation and subsequent ISRE activation. These results demonstrate that PPAR-γ negatively regulates IFN-β production in TLR3- and 4-stimulated macrophages by preventing IRF3 binding to the IFN-β promoter.
Figures

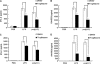

Similar articles
-
LY294002 inhibits TLR3/4-mediated IFN-β production via inhibition of IRF3 activation with a PI3K-independent mechanism.FEBS Lett. 2012 Mar 23;586(6):705-10. doi: 10.1016/j.febslet.2012.01.016. Epub 2012 Jan 26. FEBS Lett. 2012. PMID: 22285490
-
Identification of TBK1 complexes required for the phosphorylation of IRF3 and the production of interferon β.Biochem J. 2017 Mar 15;474(7):1163-1174. doi: 10.1042/BCJ20160992. Biochem J. 2017. PMID: 28159912 Free PMC article.
-
Zinc finger protein 64 promotes Toll-like receptor-triggered proinflammatory and type I interferon production in macrophages by enhancing p65 subunit activation.J Biol Chem. 2013 Aug 23;288(34):24600-8. doi: 10.1074/jbc.M113.473397. Epub 2013 Jul 15. J Biol Chem. 2013. Retraction in: J Biol Chem. 2021 Jan-Jun;296:100756. doi: 10.1016/j.jbc.2021.100756. PMID: 23857586 Free PMC article. Retracted.
-
IRF3 function and immunological gaps in sepsis.Front Immunol. 2024 Feb 5;15:1336813. doi: 10.3389/fimmu.2024.1336813. eCollection 2024. Front Immunol. 2024. PMID: 38375470 Free PMC article. Review.
-
Peroxisome proliferator-activated receptor gamma (PPAR-γ) and neurodegenerative disorders.Mol Neurobiol. 2012 Aug;46(1):114-24. doi: 10.1007/s12035-012-8259-8. Epub 2012 Mar 21. Mol Neurobiol. 2012. PMID: 22434581 Review.
Cited by
-
Activation and regulation of interferon-β in immune responses.Immunol Res. 2012 Sep;53(1-3):25-40. doi: 10.1007/s12026-012-8293-7. Immunol Res. 2012. PMID: 22411096 Review.
-
Inhibition of tumor growth by a newly-identified activator for epidermal fatty acid binding protein.Oncotarget. 2015 Apr 10;6(10):7815-27. doi: 10.18632/oncotarget.3485. Oncotarget. 2015. PMID: 25796556 Free PMC article.
-
PPARγ Deficiency Suppresses the Release of IL-1β and IL-1α in Macrophages via a Type 1 IFN-Dependent Mechanism.J Immunol. 2018 Oct 1;201(7):2054-2069. doi: 10.4049/jimmunol.1800224. Epub 2018 Aug 24. J Immunol. 2018. PMID: 30143592 Free PMC article.
-
Effects of eicosapentaneoic acid on innate immune responses in an Atlantic salmon kidney cell line in vitro.PLoS One. 2024 May 28;19(5):e0302286. doi: 10.1371/journal.pone.0302286. eCollection 2024. PLoS One. 2024. PMID: 38805503 Free PMC article.
-
Negative regulatory approaches to the attenuation of Toll-like receptor signaling.Exp Mol Med. 2013 Feb 22;45(2):e11. doi: 10.1038/emm.2013.28. Exp Mol Med. 2013. PMID: 23429360 Free PMC article. Review.
References
-
- Kawai T., Akira S. (2006) Nat. Immunol. 7, 131–137 - PubMed
-
- Hertzog P. J., O'Neill L. A., Hamilton J. A. (2003) Trends Immunol. 24, 534–539 - PubMed
-
- Kenny E. F., O'Neill L. A. (2008) Cytokine 43, 342–349 - PubMed
-
- Banchereau J., Pascual V. (2006) Immunity 25, 383–392 - PubMed
-
- Mangini A. J., Lafyatis R., Van Seventer J. M. (2007) Ann. N.Y. Acad. Sci. 1108, 11–23 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

