Mechanism of melibiose/cation symport of the melibiose permease of Salmonella typhimurium
- PMID: 21148559
- PMCID: PMC3057838
- DOI: 10.1074/jbc.M110.206227
Mechanism of melibiose/cation symport of the melibiose permease of Salmonella typhimurium
Abstract
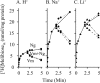
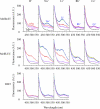
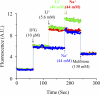
The MelB permease of Salmonella typhimurium (MelB-ST) catalyzes the coupled symport of melibiose and Na(+), Li(+), or H(+). In right-side-out membrane vesicles, melibiose efflux is inhibited by an inwardly directed gradient of Na(+) or Li(+) and stimulated by equimolar concentrations of internal and external Na(+) or Li(+). Melibiose exchange is faster than efflux in the presence of H(+) or Na(+) and stimulated by an inwardly directed Na(+) gradient. Thus, sugar is released from MelB-ST externally prior to the release of cation in agreement with current models proposed for MelB of Escherichia coli (MelB-EC) and LacY. Although Li(+) stimulates efflux, and an outwardly directed Li(+) gradient increases exchange, it is striking that internal and external Li(+) with no gradient inhibits exchange. Furthermore, Trp → dansyl FRET measurements with a fluorescent sugar (2'-(N-dansyl)aminoalkyl-1-thio-β-D-galactopyranoside) demonstrate that MelB-ST, in the presence of Na(+) or Li(+), exhibits (app)K(d) values of ∼1 mM for melibiose. Na(+) and Li(+) compete for a common binding pocket with activation constants for FRET of ∼1 mM, whereas Rb(+) or Cs(+) exhibits little or no effect. Taken together, the findings indicate that MelB-ST utilizes H(+) in addition to Na(+) and Li(+). FRET studies also show symmetrical emission maximum at ∼500 nm with MelB-ST in the presence of 2'-(N-dansyl)aminoalkyl-1-thio-β-D-galactopyranoside and Na(+), Li(+), or H(+), which implies a relatively homogeneous distribution of conformers of MelB-ST ternary complexes in the membrane.
Figures



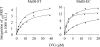
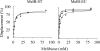
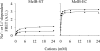
References
-
- Smith K. M., Slugoski M. D., Loewen S. K., Ng A. M., Yao S. Y., Chen X. Z., Karpinski E., Cass C. E., Baldwin S. A., Young J. D. (2005) J. Biol. Chem. 280, 25436–25449 - PubMed
-
- Hirayama B. A., Loo D. D., Wright E. M. (1997) J. Biol. Chem. 272, 2110–2115 - PubMed
-
- Wilson D. M., Wilson T. H. (1987) Biochim. Biophys. Acta 904, 191–200 - PubMed
-
- Saier M. H., Jr. (2000) Mol. Microbiol. 35, 699–710 - PubMed
-
- Kaback H. R., Sahin-Tóth M., Weinglass A. B. (2001) Nat. Rev. Mol. Cell Biol. 2, 610–620 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

