Approval summary: erlotinib maintenance therapy of advanced/metastatic non-small cell lung cancer (NSCLC)
- PMID: 21148614
- PMCID: PMC3227916
- DOI: 10.1634/theoncologist.2010-0257
Approval summary: erlotinib maintenance therapy of advanced/metastatic non-small cell lung cancer (NSCLC)
Abstract
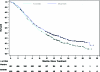
On April 16, 2010, the U. S. Food and Drug Administration (FDA) approved erlotinib tablets (Tarceva®; OSI Pharmaceuticals, Inc., Melville, NY) for maintenance treatment of patients with stage IIIB/IV non-small cell lung cancer (NSCLC) whose disease had not progressed after four cycles of platinum-based first-line chemotherapy. In total, 889 patients received either erlotinib (150 mg) or placebo once daily. Progression-free survival (PFS), in all patients and in patients with epidermal growth factor receptor (EGFR)(+) tumors by immunohistochemistry (IHC), was the primary efficacy endpoint. Overall survival (OS) was a secondary sponsor endpoint but was the primary regulatory endpoint. Median PFS times were 2.8 months and 2.6 months in the erlotinib and placebo arms, respectively (hazard ratio [HR], 0.71; 95% confidence interval [CI], 0.62-0.82; p < .001). Median OS times were 12.0 months and 11.0 months, favoring erlotinib (HR, 0.81; 95% CI, 0.70-0.95). The PFS and OS HRs in patients with EGFR(+) tumors by IHC were 0.69 (95% CI, 0.58-0.82) and 0.77 (95% CI, 0.64-0.93), respectively. The PFS and OS HRs in patients with EGFR(-) tumors by IHC were 0.77 (95% CI, 0.51-1.14) and 0.91 (95% CI, 0.59-1.38), respectively. Following disease progression, 57% of placebo-treated patients received additional chemotherapy, compared with 47% of erlotinib-treated patients. Fourteen percent of placebo-treated patients received erlotinib or gefitinib, 31% received docetaxel, and 14% received pemetrexed. In total, 59% of placebo-treated patients who received treatment received FDA approved second-line NSCLC drugs. The most common adverse reactions in patients receiving erlotinib were rash and diarrhea.
Conflict of interest statement
Section Editor
Section Editor
Reviewer “A” discloses research funding from AstraZeneca, Lilly, Pfizer, and ImClone.
Reviewer “B” discloses a consulting relationship with Genentech and honoraria received from Genentech and Lilly.
The content of this article has been reviewed by independent peer reviewers to ensure that it is balanced, objective, and free from commercial bias. On the basis of disclosed information, all conflicts of interest have been resolved.
Figures
Similar articles
-
U.S. Food and Drug Administration approval summary: Erlotinib for the first-line treatment of metastatic non-small cell lung cancer with epidermal growth factor receptor exon 19 deletions or exon 21 (L858R) substitution mutations.Oncologist. 2014 Jul;19(7):774-9. doi: 10.1634/theoncologist.2014-0089. Epub 2014 May 27. Oncologist. 2014. PMID: 24868098 Free PMC article.
-
FDA drug approval summary: erlotinib (Tarceva) tablets.Oncologist. 2005 Aug;10(7):461-6. doi: 10.1634/theoncologist.10-7-461. Oncologist. 2005. PMID: 16079312 Clinical Trial.
-
A randomized, double-blind, phase II study of erlotinib with or without sunitinib for the second-line treatment of metastatic non-small-cell lung cancer (NSCLC).Ann Oncol. 2013 Sep;24(9):2382-9. doi: 10.1093/annonc/mdt212. Epub 2013 Jun 20. Ann Oncol. 2013. PMID: 23788751 Free PMC article. Clinical Trial.
-
Effect of smoking status on progression-free and overall survival in non-small cell lung cancer patients receiving erlotinib or gefitinib: a meta-analysis.J Clin Pharm Ther. 2015 Dec;40(6):661-71. doi: 10.1111/jcpt.12332. Epub 2015 Nov 17. J Clin Pharm Ther. 2015. PMID: 26573867
-
Gefitinib or Erlotinib vs Chemotherapy for EGFR Mutation-Positive Lung Cancer: Individual Patient Data Meta-Analysis of Overall Survival.J Natl Cancer Inst. 2017 Jun 1;109(6). doi: 10.1093/jnci/djw279. J Natl Cancer Inst. 2017. PMID: 28376144 Review.
Cited by
-
Increased expression of epidermal growth factor receptor (EGF-R) in patients with different forms of lung fibrosis.Biomed Res Int. 2013;2013:654354. doi: 10.1155/2013/654354. Epub 2013 Jun 9. Biomed Res Int. 2013. PMID: 23841084 Free PMC article.
-
Delivery of RNAi Therapeutics to the Airways-From Bench to Bedside.Molecules. 2016 Sep 20;21(9):1249. doi: 10.3390/molecules21091249. Molecules. 2016. PMID: 27657028 Free PMC article. Review.
-
How and when to use genetic markers for nonsmall cell lung cancer.Curr Opin Pulm Med. 2013 Jul;19(4):331-9. doi: 10.1097/MCP.0b013e328362075c. Curr Opin Pulm Med. 2013. PMID: 23715289 Free PMC article. Review.
-
EGFR first- and second-generation TKIs-there is still place for them in EGFR-mutant NSCLC patients.Transl Cancer Res. 2019 Jan;8(Suppl 1):S23-S47. doi: 10.21037/tcr.2018.10.06. Transl Cancer Res. 2019. PMID: 35117062 Free PMC article. Review.
-
Single-agent maintenance therapy for advanced non-small cell lung cancer (NSCLC): a systematic review and Bayesian network meta-analysis of 26 randomized controlled trials.PeerJ. 2016 Oct 20;4:e2550. doi: 10.7717/peerj.2550. eCollection 2016. PeerJ. 2016. PMID: 27781159 Free PMC article.
References
-
- Gandara DR, Mack PC, Li T, et al. Evolving treatment algorithms for advanced non-small-cell lung cancer: 2009 looking toward 2012. Clin Lung Cancer. 2009;10:392–394. - PubMed
-
- Stinchcombe TE, Socinski MA. Treatment paradigms for advanced stage non-small cell lung cancer in the era of multiple lines of therapy. J Thorac Oncol. 2009;4:243–250. - PubMed
-
- Peterson P, Park K, Fossella F, et al. Is pemetrexed more effective in adenocarcinoma and large cell lung cancer than in squamous cell carcinoma? A retrospective analysis of a phase III trial of pemetrexed vs docetaxel in previously treated patients with advanced non-small cell lung cancer (NSCLC) J Thorac Oncol. 2007;2(suppl 4):s316–s317.
-
- Scagliotti G, Hanna N, Fossella F, et al. The differential efficacy of pemetrexed according to NSCLC histology: A review of two phase III studiesr. The Oncologist. 2009;14:253–263. - PubMed
-
- Ceppi P, Volante M, Saviozzi S, et al. Squamous cell carcinoma of the lung compared with other histotypes shows higher messenger RNA and protein levels for thymidylate synthase. Cancer. 2006;107:1589–1596. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous