A central role for CD68(+) macrophages in hepatopulmonary syndrome. Reversal by macrophage depletion
- PMID: 21148721
- PMCID: PMC3086745
- DOI: 10.1164/rccm.201008-1303OC
A central role for CD68(+) macrophages in hepatopulmonary syndrome. Reversal by macrophage depletion
Abstract
Rationale: The etiology of hepatopulmonary syndrome (HPS), a common complication of cirrhosis, is unknown. Inflammation and macrophage accumulation occur in HPS; however, their importance is unclear. Common bile duct ligation (CBDL) creates an accepted model of HPS, allowing us to investigate the cause of HPS.
Objectives: We hypothesized that macrophages are central to HPS and investigated the therapeutic potential of macrophage depletion.
Methods: Hemodynamics, alveolar-arterial gradient, vascular reactivity, and histology were assessed in CBDL versus sham rats (n = 21 per group). The effects of plasma on smooth muscle cell proliferation and endothelial tube formation were measured. Macrophage depletion was used to prevent (gadolinium) or regress (clodronate) HPS. CD68(+) macrophages and capillary density were measured in the lungs of patients with cirrhosis versus control patients (n = 10 per group).
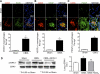
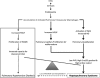
Measurements and main results: CBDL increased cardiac output and alveolar-arterial gradient by causing capillary dilatation and arteriovenous malformations. Activated CD68(+)macrophages (nuclear factor-κB+) accumulated in HPS pulmonary arteries, drawn by elevated levels of plasma endotoxin and lung monocyte chemoattractant protein-1. These macrophages expressed inducible nitric oxide synthase, vascular endothelial growth factor, and platelet-derived growth factor. HPS plasma increased endothelial tube formation and pulmonary artery smooth muscle cell proliferation. Macrophage depletion prevented and reversed the histological and hemodynamic features of HPS. CBDL lungs demonstrated increased medial thickness and obstruction of small pulmonary arteries. Nitric oxide synthase inhibition unmasked exaggerated pulmonary vasoconstrictor responses in HPS. Patients with cirrhosis had increased pulmonary intravascular macrophage accumulation and capillary density.
Conclusions: HPS results from intravascular accumulation of CD68(+)macrophages. An occult proliferative vasculopathy may explain the occasional transition to portopulmonary hypertension. Macrophage depletion may have therapeutic potential in HPS.
Figures







References
-
- Rodriguez-Roisin R, Krowka MJ. Hepatopulmonary syndrome—a liver-induced lung vascular disorder. N Engl J Med 2008;358:2378–2387. - PubMed
-
- Rodriguez-Roisin R, Krowka MJ, Herve P, Fallon MB. Pulmonary-hepatic vascular disorders (PHD). Eur Respir J 2004;24:861–880. - PubMed
-
- Aucejo F, Miller C, Vogt D, Eghtesad B, Nakagawa S, Stoller JK. Pulmonary hypertension after liver transplantation in patients with antecedent hepatopulmonary syndrome: a report of 2 cases and review of the literature. Liver Transpl 2006;12:1278–1282. - PubMed
-
- Chang SW, Ohara N. Pulmonary circulatory dysfunction in rats with biliary cirrhosis. An animal model of the hepatopulmonary syndrome. Am Rev Respir Dis 1992;145:798–805. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

