Mitigation of chlorine gas lung injury in rats by postexposure administration of sodium nitrite
- PMID: 21148791
- PMCID: PMC3064287
- DOI: 10.1152/ajplung.00278.2010
Mitigation of chlorine gas lung injury in rats by postexposure administration of sodium nitrite
Abstract
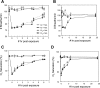
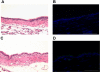
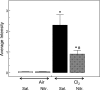
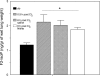
Nitrite (NO(2)(-)) has been shown to limit injury to the heart, liver, and kidneys in various models of ischemia-reperfusion injury. Potential protective effects of systemic NO(2)(-) in limiting lung injury or enhancing repair have not been documented. We assessed the efficacy and mechanisms by which postexposure intraperitoneal injections of NO(2)(-) mitigate chlorine (Cl(2))-induced lung injury in rats. Rats were exposed to Cl(2) (400 ppm) for 30 min and returned to room air. NO(2)(-) (1 mg/kg) or saline was administered intraperitoneally at 10 min and 2, 4, and 6 h after exposure. Rats were killed at 6 or 24 h. Injury to airway and alveolar epithelia was assessed by quantitative morphology, protein concentrations, number of cells in bronchoalveolar lavage (BAL), and wet-to-dry lung weight ratio. Lipid peroxidation was assessed by measurement of lung F(2)-isoprostanes. Rats developed severe, but transient, hypoxemia. A significant increase of protein concentration, neutrophil numbers, airway epithelia in the BAL, and lung wet-to-dry weight ratio was evident at 6 h after Cl(2) exposure. Quantitative morphology revealed extensive lung injury in the upper airways. Airway epithelial cells stained positive for terminal deoxynucleotidyl-mediated dUTP nick end labeling (TUNEL), but not caspase-3. Administration of NO(2)(-) resulted in lower BAL protein levels, significant reduction in the intensity of the TUNEL-positive cells, and normal lung wet-to-dry weight ratios. F(2)-isoprostane levels increased at 6 and 24 h after Cl(2) exposure in NO(2)(-)- and saline-injected rats. This is the first demonstration that systemic NO(2)(-) administration mitigates airway and epithelial injury.
Figures
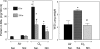



References
-
- Andonegui G, Goyert SM, Kubes P. Lipopolysaccharide-induced leukocyte-endothelial cell interactions: a role for CD14 versus toll-like receptor 4 within microvessels. J Immunol 169: 2111–2119, 2002 - PubMed
-
- Baskin SI, Horowitz AM, Nealley EW. The antidotal action of sodium nitrite and sodium thiosulfate against cyanide poisoning. J Clin Pharmacol 32: 368–375, 1992 - PubMed
-
- Bell DG. Management of acute respiratory distress syndrome (ARDS) following chlorine exposure (Abstract). Am J Respir Crit Care Med 176: A314, 2008
-
- Comellas AP, Pesce LM, Azzam Z, Saldias FJ, Sznajder JI. Scorpion venom decreases lung liquid clearance in rats. Am J Respir Crit Care Med 167: 1064–1067, 2003 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

