Exercise and pharmacotherapy in patients with major depression: one-year follow-up of the SMILE study
- PMID: 21148807
- PMCID: PMC3671874
- DOI: 10.1097/PSY.0b013e31820433a5
Exercise and pharmacotherapy in patients with major depression: one-year follow-up of the SMILE study
Abstract
Objective: To examine a 1-year follow-up of a 4-month, controlled clinical trial of exercise and antidepressant medication in patients with major depressive disorder (MDD).
Methods: In the original study, 202 sedentary adults with MDD were randomized to: a) supervised exercise; b) home-based exercise; c) sertraline; or d) placebo pill. We examined two outcomes measured at 1-year follow-up (i.e., 16 months post randomization): 1) continuous Hamilton Depression Rating Scale score; and 2) MDD status (depressed; partial remission; full remission) in 172 available participants (85% of the original cohort). Regression analyses were performed to examine the effects of treatment group assignment, as well as follow-up antidepressant medication use and self-reported exercise (Godin Leisure-Time Exercise Questionnaire), on the two outcomes.
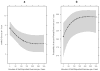
Results: In the original study, patients receiving exercise achieved similar benefits compared with those receiving sertraline. At the time of the 1-year follow-up, rates of MDD remission increased from 46% at post treatment to 66% for participants available for follow-up. Neither initial treatment group assignment nor antidepressant medication use during the follow-up period were significant predictors of MDD remission at 1 year. However, regular exercise during the follow-up period predicted both Hamilton Depression Rating Scale scores and MDD diagnosis at 1 year. This relationship was curvilinear, with the association concentrated between 0 minute and 180 minutes of weekly exercise.
Conclusion: The effects of aerobic exercise on MDD remission seem to be similar to sertraline after 4 months of treatment; exercise during the follow-up period seems to extend the short-term benefits of exercise and may augment the benefits of antidepressant use.
Trial registration: clinicaltrials.gov Identifier: NCT00331305.
Conflict of interest statement
The remaining authors did not disclose any potential conflicts of interest.
Figures
References
-
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Waters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:593–602. - PubMed
-
- Moussavi S, Chatterji S, Verdes E, Tandon A, Patel V, Uston B. Depression, chronic disease, and decrements in health: results from the World Health Survey. Lancet. 2007;370:851–8. - PubMed
-
- Hollon SD, Thase ME, Markowitz JC. Treatment and prevention of depression. Psychol Sci Public Interest. 2002;3:39–77. - PubMed
-
- Smith D, Dempster C, Glanville J, Freemantle N, Anderson I. Efficacy and tolerability of venlafaxine compared with selective serotonin re-uptake inhibitors and other antidepressants: a meta-analysis. Br J Psychiatry. 2002;180:396–404. - PubMed
-
- Rush AJ, Trivendi MH, Wisniewski SR, Nierenberg AA, Stewart JW, Warden D, Niederehe G, Thase ME, Lavori PW, Lebowitz BD, McGrath PJ, Rosenbaum JF, Sackeim HA, Kupfer DJ, Luther J, Fava M. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163:1905–17. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

