Conserved natural IgM antibodies mediate innate and adaptive immunity against the opportunistic fungus Pneumocystis murina
- PMID: 21149550
- PMCID: PMC3005228
- DOI: 10.1084/jem.20100034
Conserved natural IgM antibodies mediate innate and adaptive immunity against the opportunistic fungus Pneumocystis murina
Abstract
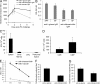
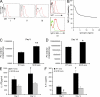
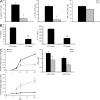
Host defense against opportunistic fungi requires coordination between innate and adaptive immunity for resolution of infection. Antibodies generated in mice vaccinated with the fungus Pneumocystis prevent growth of Pneumocystis organisms within the lungs, but the mechanisms whereby antibodies enhance antifungal host defense are poorly defined. Nearly all species of fungi contain the conserved carbohydrates β-glucan and chitin within their cell walls, which may be targets of innate and adaptive immunity. In this study, we show that natural IgM antibodies targeting these fungal cell wall carbohydrates are conserved across many species, including fish and mammals. Natural antibodies bind fungal organisms and enhance host defense against Pneumocystis in early stages of infection. IgM antibodies influence recognition of fungal antigen by dendritic cells, increasing their migration to draining pulmonary lymph nodes. IgM antibodies are required for adaptive T helper type 2 (Th2) and Th17 cell differentiation and guide B cell isotype class-switch recombination during host defense against Pneumocystis. These experiments suggest a novel role for the IgM isotype in shaping the earliest steps in recognition and clearance of this fungus. We outline a mechanism whereby serum IgM, containing ancient specificities against conserved fungal antigens, bridges innate and adaptive immunity against fungal organisms.
Figures


References
-
- Ben-Hur H., Gurevich P., Elhayany A., Avinoach I., Schneider D.F., Zusman I. 2005. Transport of maternal immunoglobulins through the human placental barrier in normal pregnancy and during inflammation. Int. J. Mol. Med. 16:401–407 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

