Infection-induced myelopoiesis during intracellular bacterial infection is critically dependent upon IFN-γ signaling
- PMID: 21149601
- PMCID: PMC3178067
- DOI: 10.4049/jimmunol.1001893
Infection-induced myelopoiesis during intracellular bacterial infection is critically dependent upon IFN-γ signaling
Abstract
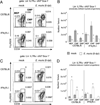
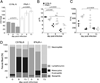
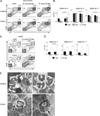
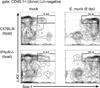
Although microbial infections can alter steady-state hematopoiesis, the mechanisms that drive such changes are not well understood. We addressed a role for IFN-γ signaling in infection-induced bone marrow suppression and anemia in a murine model of human monocytic ehrlichiosis, an emerging tick-borne disease. Within the bone marrow of Ehrlichia muris-infected C57BL/6 mice, we observed a reduction in myeloid progenitor cells, as defined both phenotypically and functionally. Infected mice exhibited a concomitant increase in developing myeloid cells within the bone marrow, an increase in the frequency of circulating monocytes, and an increase in splenic myeloid cells. The infection-induced changes in progenitor cell phenotype were critically dependent on IFN-γ, but not IFN-α, signaling. In mice deficient in the IFN-γ signaling pathway, we observed an increase in myeloid progenitor cells and CDllb(lo)Gr1(lo) promyelocytic cells within the bone marrow, as well as reduced frequencies of mature granulocytes and monocytes. Furthermore, E. muris-infected IFN-γR-deficient mice did not exhibit anemia or an increase in circulating monocytes, and they succumbed to infection. Gene transcription studies revealed that IFN-γR-deficient CDllb(lo)Gr1(lo) promyelocytes from E. muris-infected mice exhibited significantly reduced expression of irf-1 and irf-8, both key transcription factors that regulate the differentiation of granulocytes and monocytes. Finally, using mixed bone marrow chimeric mice, we show that IFN-γ-dependent infection-induced myelopoiesis occurs via the direct effect of the cytokine on developing myeloid cells. We propose that, in addition to its many other known roles, IFN-γ acts to control infection by directly promoting the differentiation of myeloid cells that contribute to host defense.
Conflict of interest statement
The authors have no financial conflicts of interest.
Figures



References
-
- Yáñez A, Murciano C, O’Connor JE, Gozalbo D, Gil ML. Candida albicans triggers proliferation and differentiation of hematopoietic stem and progenitor cells by a MyD88-dependent signaling. Microbes Infect. 2009;11:531–535. - PubMed
-
- Basu S, Hodgson G, Zhang HH, Katz M, Quilici C, Dunn AR. “Emergency” granulopoiesis in G-CSF-deficient mice in response to Candida albicans infection. Blood. 2000;95:3725–3733. - PubMed
-
- Burg ND, Pillinger MH. The neutrophil: function and regulation in innate and humoral immunity. Clin. Immunol. 2001;99:7–17. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

