Structural space of protein-protein interfaces is degenerate, close to complete, and highly connected
- PMID: 21149688
- PMCID: PMC3012513
- DOI: 10.1073/pnas.1012820107
Structural space of protein-protein interfaces is degenerate, close to complete, and highly connected
Abstract
At the heart of protein-protein interactions are protein-protein interfaces where the direct physical interactions occur. By developing and applying an efficient structural alignment method, we study the structural similarity of representative protein-protein interfaces involving interactions between dimers. Even without structural similarity between individual monomers that form dimeric complexes, ∼90% of native interfaces have a close structural neighbor with similar backbone C(α) geometry and interfacial contact pattern. About 80% of the interfaces form a dense network, where any two interfaces are structurally related using a transitive set of at most seven intermediate interfaces. The degeneracy of interface space is largely due to the packing of compact, hydrogen-bonded secondary structure elements. This packing generates relatively flat interacting surfaces whose geometries are highly degenerate. Comparative study of artificial and native interfaces argues that the library of protein interfaces is close to complete and comprised of roughly 1,000 distinct interface types. In contrast, the number of possible quaternary structures of dimers is estimated to be about 10(4) times larger; thus, an experimentally determined database of all representative quaternary structures is not likely in the near future. Nevertheless, one could in principle exploit the completeness of protein interfaces to predict most dimeric quaternary structures. Finally, our results provide a structural explanation for the prevalence of promiscuous protein interactions. By side-chain packing adjustments, we illustrate how multiprotein specificity can be attained at a promiscuous interface.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
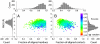

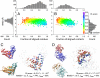



References
-
- Russell RB, et al. A structural perspective on protein–protein interactions. Curr Opin Struct Biol. 2004;14:313–324. - PubMed
-
- Janin J, Bahadur RP, Chakrabarti P. Protein–protein interaction and quaternary structure. Q Rev Biophys. 2008;41:133–180. - PubMed
-
- Keskin Z, Gursoy A, Ma B, Nussinov R. Principles of protein–protein interactions: What are the preferred ways for proteins to interact? Chem Rev. 2008;108:1225–1244. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

