Spatial restriction of receptor tyrosine kinase activity through a polarized endocytic cycle controls border cell migration
- PMID: 21149700
- PMCID: PMC3012526
- DOI: 10.1073/pnas.1010795108
Spatial restriction of receptor tyrosine kinase activity through a polarized endocytic cycle controls border cell migration
Abstract
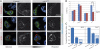
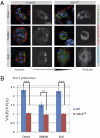
Border cell migration is a stereotyped migration occurring during the development of the Drosophila egg chamber. During this process, a cluster composed of six to eight follicle cells migrates between nurse cells toward the oocyte. Receptor tyrosine kinases (RTKs) are enriched at the leading edge of the follicle cells and establish the directionality of their migration. Endocytosis has been shown to play a role in the maintenance of this polarization; however, the mechanisms involved are largely unknown. In this study, we show that border cell migration requires the function of the small GTPases Rab5 and Rab11 that regulate trafficking through the early and the recycling endosome, respectively. Expression of a dominant negative form of rab11 induces a loss of the polarization of RTK activity, which correlates with a severe migration phenotype. In addition, we demonstrate that the exocyst component Sec15 is distributed in structures that are polarized during the migration process in a Rab11-dependent manner and that the down-regulation of different subunits of the exocyst also affects migration. Together, our data demonstrate a fundamental role for a plasma membrane-endosome trafficking cycle in the maintenance of active RTK at the leading edge of border cells during their migration.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Montell DJ. Morphogenetic cell movements: Diversity from modular mechanical properties. Science. 2008;322:1502–1505. - PubMed
-
- Rørth P. Collective guidance of collective cell migration. Trends Cell Biol. 2007;17:575–579. - PubMed
-
- Rørth P. Initiating and guiding migration: Lessons from border cells. Trends Cell Biol. 2002;12:325–331. - PubMed
-
- Doherty GJ, McMahon HT. Mechanisms of endocytosis. Annu Rev Biochem. 2009;78:857–902. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

