Inactivation of p53 in breast cancers correlates with stem cell transcriptional signatures
- PMID: 21149740
- PMCID: PMC3012457
- DOI: 10.1073/pnas.1017001108
Inactivation of p53 in breast cancers correlates with stem cell transcriptional signatures
Abstract
Breast cancer comprises a heterogeneous set of diseases distinguishable from one another by pathologic presentation and molecular signatures. However, each breast cancer subtype is also heterogeneous. Some of the heterogeneity may be attributable to genetic instability, but recent data emphasize that developmental plasticity may also contribute. The p53 tumor suppressor could constitute a nodal control point underlying both sources of heterogeneity because it is frequently inactivated during malignant progression and has recently been shown to function as a potent barrier preventing fully differentiated cells from reverting to pluripotent stem cells after expression of appropriate oncogenes. Using archival microarray datasets, we tested the hypothesis that a p53 mutation could allow cells within a tumor to acquire a stem cell-like state by looking for coordinate expression of stem cell identity genes. We show that breast and lung cancers with p53 mutations do exhibit stem cell-like transcriptional patterns. Such tumors were also depleted for differentiation genes regulated by the polycomb repressor complex 2. These data are consistent with a model in which loss of p53 function enables acquisition of stem cell properties, which are positively selected during tumor progression.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

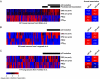
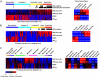
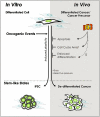
References
-
- Merlo LM, Pepper JW, Reid BJ, Maley CC. Cancer as an evolutionary and ecological process. Nat Rev Cancer. 2006;6:924–935. - PubMed
-
- Vogelstein B, Kinzler KW. The multistep nature of cancer. Trends Genet. 1993;9:138–141. - PubMed
-
- Stingl J, Caldas C. Molecular heterogeneity of breast carcinomas and the cancer stem cell hypothesis. Nat Rev Cancer. 2007;7:791–799. - PubMed
-
- Gupta PB, Chaffer CL, Weinberg RA. Cancer stem cells: Mirage or reality? Nat Med. 2009;15:1010–1012. - PubMed
-
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

