Electronic patient messages to promote colorectal cancer screening: a randomized controlled trial
- PMID: 21149743
- PMCID: PMC3169179
- DOI: 10.1001/archinternmed.2010.467
Electronic patient messages to promote colorectal cancer screening: a randomized controlled trial
Abstract
Background: Colorectal cancer is a leading cause of cancer mortality, yet effective screening tests are often underused. Electronic patient messages and personalized risk assessments delivered via an electronic personal health record could increase screening rates.
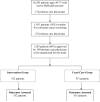
Methods: We conducted a randomized controlled trial in 14 ambulatory health centers involving 1103 patients ranging in age from 50 to 75 years with an active electronic personal health record who were overdue for colorectal cancer screening. Patients were randomly assigned to receive a single electronic message highlighting overdue screening status with a link to a Web-based tool to assess their personal risk of colorectal cancer. The outcomes included colorectal cancer screening rates at 1 and 4 months.
Results: Screening rates were higher at 1 month for patients who received electronic messages than for those who did not (8.3% vs 0.2%, P < .001), but this difference was no longer significant at 4 months (15.8% vs 13.1%, P = .18). Of 552 patients randomized to receive the intervention, 296 (54%) viewed the message, and 47 (9%) used the Web-based risk assessment tool. Among 296 intervention patients who viewed the electronic message, risk tool users were more likely than nonusers to request screening examinations (17% vs 4%, P = .04) and to be screened (30% vs 15%, P = .06). One-fifth of patients (19%) using the risk assessment tool were estimated to have an above-average risk for colorectal cancer.
Conclusion: Electronic messages to patients produce an initial increase in colorectal cancer screening rates, but this effect is not sustained over time.
Trial registration: clinicaltrials.gov Identifier: NCT01032746.
Figures
References
-
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. - PubMed
-
- Mandel JS, Bond JH, Church TR, et al. Reducing mortality from colorectal cancer by screening for fecal occult blood. Minnesota Colon Cancer Control Study. N Engl J Med. 1993;328:1365–1371. - PubMed
-
- Hardcastle JD, Chamberlain JO, Robinson MH, et al. Randomised controlled trial of faecal-occult-blood screening for colorectal cancer. Lancet. 1996;348:1472–1477. - PubMed
-
- Kronborg O, Fenger C, Olsen J, Jorgensen OD, Sondergaard O. Randomised study of screening for colorectal cancer with faecal-occult-blood test. Lancet. 1996;348:1467–1471. - PubMed
-
- Selby JV, Friedman GD, Quesenberry CP, Jr., Weiss NS. A case-control study of screening sigmoidoscopy and mortality from colorectal cancer. N Engl J Med. 1992;326:653–657. - PubMed