LIF-independent JAK signalling to chromatin in embryonic stem cells uncovered from an adult stem cell disease
- PMID: 21151131
- PMCID: PMC3008749
- DOI: 10.1038/ncb2135
LIF-independent JAK signalling to chromatin in embryonic stem cells uncovered from an adult stem cell disease
Abstract
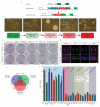
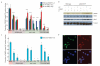
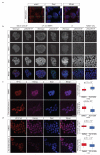
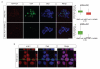
Activating mutations in the tyrosine kinase Janus kinase 2 (JAK2) cause myeloproliferative neoplasms, clonal blood stem cell disorders with a propensity for leukaemic transformation. Leukaemia inhibitory factor (LIF) signalling through the JAK-signal transducer and activator of transcription (STAT) pathway enables self-renewal of embryonic stem (ES) cells. Here we show that mouse ES cells carrying the human JAK2V617F mutation were able to self-renew in chemically defined conditions without cytokines or small-molecule inhibitors, independently of JAK signalling through the STAT3 or phosphatidylinositol-3-OH kinase pathways. Phosphorylation of histone H3 tyrosine 41 (H3Y41) by JAK2 was recently shown to interfere with binding of heterochromatin protein 1α (HP1α). Levels of chromatin-bound HP1α were lower in JAK2V617F ES cells but increased following inhibition of JAK2, coincident with a global reduction in histone H3Y41 phosphorylation. JAK2 inhibition reduced levels of the pluripotency regulator Nanog, with a reduction in H3Y41 phosphorylation and concomitant increase in HP1α levels at the Nanog promoter. Furthermore, Nanog was required for factor independence of JAK2V617F ES cells. Taken together, these results uncover a previously unrecognized role for direct signalling to chromatin by JAK2 as an important mediator of ES cell self-renewal.
Figures


References
-
- Campbell PJ, Green AR. The myeloproliferative disorders. N. Engl. J. Med. 2006;355:2452–2466. - PubMed
-
- James C, et al. A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature. 2005;434:1144–1148. - PubMed
-
- Levine RL, et al. Activating mutation in the tyrosine kinase JAK2 in polycythemia vera, essential thrombocythemia, and myeloid metaplasia with myelofibrosis. Cancer Cell. 2005;7:387–397. - PubMed
-
- Baxter EJ, et al. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet. 2005;365:1054–1061. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- G0900729/1/NC3RS_/National Centre for the Replacement, Refinement and Reduction of Animals in Research/United Kingdom
- G0800784/MRC_/Medical Research Council/United Kingdom
- A8043/CRUK_/Cancer Research UK/United Kingdom
- G0600275/MRC_/Medical Research Council/United Kingdom
- G1000847/MRC_/Medical Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

