Analysis of agonist-antagonist interactions at A1 adenosine receptors
- PMID: 2115114
- PMCID: PMC5567770
Analysis of agonist-antagonist interactions at A1 adenosine receptors
Abstract
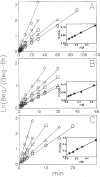
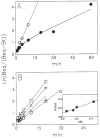
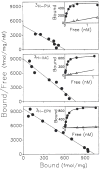
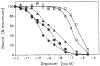
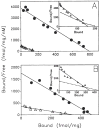
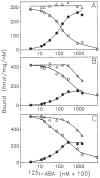
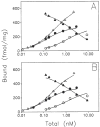
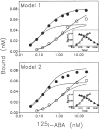
Previous work from our laboratory using sucrose gradient centrifugation and the antagonist radioligand [3H]xanthine amine congener led us to propose that A1 adenosine receptors are coupled to a GTP-binding protein (G protein) in the absence of an agonist and that adenosine receptor antagonists bind to free uncoupled receptors with high affinity and coupled receptors with low affinity and cause a destabilization of receptor-G protein complexes [Mol. Pharmacol. 36:412-419 (1989)]. Because agonists form high affinity ternary complexes composed of the agonist, receptor, and G protein, this hypothesis would imply that interactions between adenosine receptor agonists and antagonists, while competitive, would appear to be "noncompetitive" in nature. Interactions between unlabeled and radiolabeled A1 receptor agonist and antagonist ligands have been investigated using bovine cerebral cortical membranes to further probe this point. The availability of both 3H- and 125I-radioligands allowed us to use both single- and dual-isotope experimental designs. Radioligand antagonist-agonist competition curves along with saturation analyses using filtration and centrifugation to isolate bound radioligand suggested that agonists bind to two sites or receptor states with high affinity and to one site with low affinity. Agonist radioligand saturation curves with or without unlabeled antagonist suggested that antagonists do not bind to all states of the receptor with equal affinity. The computer program EQUIL was used to define models capable of simultaneously fitting all parts of complex experiments in which 125I-N6-aminobenzyladenosine saturation isotherms with or without 8-cyclopentyl-1,3-dipropylxanthine ([3H]CPX) and a saturation isotherm of [3H]CPX were performed. The data were not compatible with two-independent site models or with ternary complex models involving one receptor and one G protein. The data were fit by a model involving one receptor and two G proteins and by a model involving two receptors and one G protein. Both models suggest that 1) a high percentage of the receptor(s) is coupled to a G protein in the absence of an agonist and 2) agonists stabilize whereas antagonists destabilize precoupled receptor-G protein complexes. Because of this, competitive interactions between A1 agonists and antagonists appear noncompetitive in nature.
Figures



References
-
- Williams M, Jacobson KA. Radioligand binding assays for adenosine receptors. In: Williams M, editor. Adenosine Receptors. Human; Clifton, NJ: in press.
-
- Jarvis MF. Autoradiographic localization and characterization of brain adenosine receptor subtypes. In: Leslie FM, Altar CA, editors. Receptor Localization: Ligand Autoradiography. Alan R Liss; New York: 1988. pp. 96–111.
-
- Schwabe U, Trost T. Characterization of adenosine receptors in rat brain by (−)[3H]N6-phenylisopropyladenosine. Naunyn-Schmiedeberg’s Arch Pharmacol. 1980;313:179–187. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
