Autophagy is essential to suppress cell stress and to allow BCR-Abl-mediated leukemogenesis
- PMID: 21151168
- PMCID: PMC3081401
- DOI: 10.1038/onc.2010.561
Autophagy is essential to suppress cell stress and to allow BCR-Abl-mediated leukemogenesis
Abstract
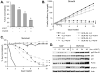
Hematopoietic cells normally require cell extrinsic signals to maintain metabolism and survival. In contrast, cancer cells can express constitutively active oncogenic kinases such as BCR-Abl that promote these processes independent of extrinsic growth factors. When cells receive insufficient growth signals or when oncogenic kinases are inhibited, glucose metabolism decreases and the self-digestive process of autophagy is elevated to degrade bulk cytoplasm and organelles. Although autophagy has been proposed to provide a cell-intrinsic nutrient supply for mitochondrial oxidative metabolism and to maintain cellular homeostasis through degradation of damaged organelles or protein aggregates, its acute role in growth factor deprivation or inhibition of oncogenic kinases remains poorly understood. We therefore developed a growth factor-dependent hematopoietic cell culture model in which autophagy can be acutely disrupted through conditional Cre-mediated excision of the autophagy-essential gene Atg3. Treated cells rapidly lost their ability to perform autophagy and underwent cell cycle arrest and apoptosis. Although Atg3 was essential for optimal upregulation of mitochondrial oxidative pathways in growth factor withdrawal, this metabolic contribution of autophagy did not appear critical for cell survival, as provision of exogenous pyruvate or lipids could not completely rescue Atg3 deficiency. Instead, autophagy suppressed a stress response that otherwise led to p53 phosphorylation and upregulation of p21 and the pro-apoptotic Bcl-2 family protein Puma. Importantly, BCR-Abl-expressing cells had low basal levels of autophagy, but were highly dependent on this process, and rapidly underwent apoptosis upon disruption of autophagy through Atg3 deletion or treatment with chemical autophagy inhibitors. This dependence on autophagy extended in vivo, as Atg3 deletion also prevented BCR-Abl-mediated leukemogenesis in a cell transfer model. Together these data demonstrate a critical role for autophagy to mitigate cell stress, and that cells expressing the oncogenic kinase BCR-Abl appear particularly dependent on autophagy for cell survival and leukemogenesis.
Figures



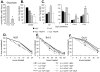
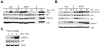



References
-
- An J, Muoio DM, Shiota M, Fujimoto Y, Cline GW, Shulman GI, et al. Hepatic expression of malonyl-CoA decarboxylase reverses muscle, liver and whole-animal insulin resistance. Nat Med. 2004;10:268–274. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

