Beyond chemotherapy: new agents for targeted treatment of lymphoma
- PMID: 21151205
- PMCID: PMC3192435
- DOI: 10.1038/nrclinonc.2010.189
Beyond chemotherapy: new agents for targeted treatment of lymphoma
Erratum in
- Nat Rev Clin Oncol. 2011 Mar;8(3):124
Abstract
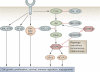
An improved understanding of the molecular biology of cancer cell growth and survival and the role of the microenvironment in supporting the survival of cancer cells, including lymphoma cells, has led to the identification of a number of potential therapeutic targets. Despite these advances, drug development for lymphoma remains slow, inefficient, and frequently unfocused. Future work should focus on identifying 'driver' molecular defects of oncogenic pathways that can be targeted therapeutically, discovering predictive biomarkers for treatment response, and prioritizing promising drugs to accelerate their approval. This Review summarizes the current development status of novel agents for lymphoma and discusses strategies to move the field forward.
Figures


References
-
- Jemal A, Center MM, Ward E, Thun MJ. Cancer occurrence. Methods Mol. Biol. 2009;471:3–29. - PubMed
-
- Zelenetz AD, et al. NCCN Clinical Practice Guidelines in Oncology: non-Hodgkin's Lymphomas. J. Natl Compr. Canc. Netw. 2010;8:288–334. - PubMed
-
- Sridhara R, et al. Review of oncology and hematology drug product approvals at the US Food and Drug Administration between July 2005 and December 2007. J. Natl Cancer Inst. 2010;102:230–243. - PubMed
-
- Cheson BD, Leonard JP. Monoclonal antibody therapy for B-cell non-Hodgkin's lymphoma. N. Engl. J. Med. 2008;359:613–626. - PubMed
-
- Coiffier B, et al. Safety and efficacy of ofatumumab, a fully human monoclonal anti-CD20 antibody, in patients with relapsed or refractory B-cell chronic lymphocytic leukemia: a phase 1–2 study. Blood. 2008;111:1094–1100. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

