Epithelial microfilament regulators show regional distribution in mouse conjunctiva
- PMID: 21151337
- PMCID: PMC2994345
Epithelial microfilament regulators show regional distribution in mouse conjunctiva
Abstract
Purpose: The conjunctival epithelium is a continuous sheet of cells with regional characteristics that appear to be similar. This study was designed to investigate the distribution and levels of expression of a subset of microfilament regulators in the forniceal, palpebral, and bulbar conjunctival epithelia.
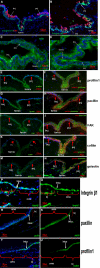
Methods: Balb/C mice were used. The localizations of paxillin, focal adhesion kinase, vinculin, talin1, cofilin, profilin, gelsolin, integrin β1, and integrin α6 were studied with the use of cross-sectional immunofluorescent staining. For a detailed cellular analysis, positioning and ablation with the laser microbeam (PALM) Combi System was used to obtain forniceal, bulbar, and palpebral conjunctival epithelia for expression comparison with the use of western blot analysis and quantitative real-time polymerase chain reaction.
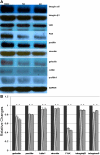
Results: Immunostaining showed that focal adhesion kinase, cofilin, profilin, gelsolin, talin1, and vinculin were expressed in all layers of the forniceal, palpebral, and bulbar conjunctival epithelia. Paxillin, integrin β1, and α6 was found to be located in the basal cell layer in all three of these areas. Quantitative real-time polymerase chain reaction showed that the transcript levels of these microfilament regulators in the forniceal conjunctivae were higher than those levels found in the bulbar and palpebral conjunctivae. Western blot analysis confirmed the differential expression levels of these microfilament regulators in the forniceal, bulbar, and palpebral conjunctivae.
Conclusions: Differences in the levels of microfilament regulators in the forniceal, bulbar, and palpebral conjunctivae suggest different modes of interaction with their microenvironment and within cell layers.
Figures

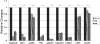
References
-
- Kurpakus MA, Maniaci MT, Esco M. Expression of keratins K12, K4 and K14 during development of ocular surface epithelium. Curr Eye Res. 1994;13:805–14. - PubMed
-
- Cotsarelis G, Cheng SZ, Dong G, Sun TT, Lavker RM. Existence of slow-cycling limbal epithelial basal cells that can be preferentially stimulated to proliferate: implications on epithelial stem cells. Cell. 1989;57:201–9. - PubMed
-
- Chen W, Ishikawa M, Yamaki K, Sakuragi S. Wistar rat palpebral conjunctiva contains more slow-cycling stem cells that have larger proliferative capacity: implication for conjunctival epithelial homeostasis. Jpn J Ophthalmol. 2003;47:119–28. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
