Face-Specific Resting Functional Connectivity between the Fusiform Gyrus and Posterior Superior Temporal Sulcus
- PMID: 21151362
- PMCID: PMC2995581
- DOI: 10.3389/fnhum.2010.00176
Face-Specific Resting Functional Connectivity between the Fusiform Gyrus and Posterior Superior Temporal Sulcus
Abstract
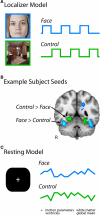
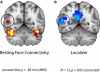
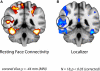
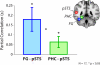
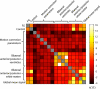
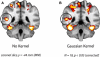
Faces activate specific brain regions in fMRI, including the fusiform gyrus (FG) and the posterior superior temporal sulcus (pSTS). The fact that the FG and pSTS are frequently co-activated suggests that they may interact synergistically in a distributed face processing network. Alternatively, the functions implemented by these regions may be encapsulated from each other. It has proven difficult to evaluate these two accounts during visual processing of face stimuli. However, if the FG and pSTS interact during face processing, the substrate for such interactions may be apparent in a correlation of the BOLD timeseries from these two regions during periods of rest when no faces are present. To examine face-specific resting correlations, we developed a new partial functional connectivity approach in which we removed variance from the FG that was shared with other category-selective and control regions. The remaining face-specific FG resting variance was then used to predict resting signals throughout the brain. In two experiments, we observed face-specific resting functional connectivity between FG and pSTS, and importantly, these correlations overlapped precisely with the face-specific pSTS region obtained from independent localizer runs. Additional region-of-interest and pattern analyses confirmed that the FG-pSTS resting correlations were face-specific. These findings support a model in which face processing is distributed among a finite number of connected, but nevertheless face-specialized regions. The discovery of category-specific interactions in the absence of visual input suggests that resting networks may provide a latent foundation for task processing.
Keywords: fMRI; face processing; functional connectivity; fusiform face area; high-level vision; inferior temporal cortex; rest; scene processing.
Figures


References
Grants and funding
LinkOut - more resources
Full Text Sources

