Identification, activity and disulfide connectivity of C-di-GMP regulating proteins in Mycobacterium tuberculosis
- PMID: 21151497
- PMCID: PMC2994820
- DOI: 10.1371/journal.pone.0015072
Identification, activity and disulfide connectivity of C-di-GMP regulating proteins in Mycobacterium tuberculosis
Abstract
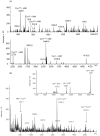
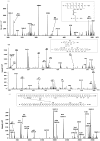
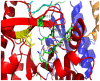
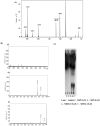
C-di-GMP, a bacterial second messenger plays a key role in survival and adaptation of bacteria under different environmental conditions. The level of c-di-GMP is regulated by two opposing activities, namely diguanylate cyclase (DGC) and phosphodiesterase (PDE-A) exhibited by GGDEF and EAL domain, respectively in the same protein. Previously, we reported a bifunctional GGDEF-EAL domain protein, MSDGC-1 from Mycobacterium smegmatis showing both these activities (Kumar and Chatterji, 2008). In this current report, we have identified and characterized the homologous protein from Mycobacterium tuberculosis (Rv 1354c) named as MtbDGC. MtbDGC is also a bifunctional protein, which can synthesize and degrade c-di-GMP in vitro. Further we expressed Mtbdgc in M. smegmatis and it was able to complement the MSDGC-1 knock out strain by restoring the long term survival of M. smegmatis. Another protein Rv 1357c, named as MtbPDE, is an EAL domain protein and degrades c-di-GMP to pGpG in vitro. Rv1354c and 1357c have seven cysteine amino acids in their sequence, distributed along the full length of the protein. Disulfide bonds play an important role in stabilizing protein structure and regulating protein function. By proteolytic digestion and mass spectrometric analysis of MtbDGC, connectivity between cysteine pairs Cys94-Cys584, Cys2-Cys479 and Cys429-Cys614 was determined, whereas the third cysteine (Cys406) from N terminal was found to be free in MtbDGC protein, which was further confirmed by alkylation with iodoacetamide labeling. Bioinformatics modeling investigations also supported the pattern of disulfide connectivity obtained by Mass spectrometric analysis. Cys406 was mutated to serine by site directed mutagenesis and the mutant MtbC406S was not found to be active and was not able to synthesize or degrade c-di-GMP. The disulfide connectivity established here would help further in understanding the structure - function relationship in MtbDGC.
Conflict of interest statement
Figures









Similar articles
-
Cyclic di-GMP: a second messenger required for long-term survival, but not for biofilm formation, in Mycobacterium smegmatis.Microbiology (Reading). 2008 Oct;154(Pt 10):2942-2955. doi: 10.1099/mic.0.2008/017806-0. Microbiology (Reading). 2008. Retraction in: Microbiology (Reading). 2011 Mar;157(Pt 3):918. doi: 10.1099/mic.0.30956-0. PMID: 18832301 Retracted.
-
Substrate-induced domain movement in a bifunctional protein, DcpA, regulates cyclic di-GMP turnover: Functional implications of a highly conserved motif.J Biol Chem. 2018 Sep 7;293(36):14065-14079. doi: 10.1074/jbc.RA118.003917. Epub 2018 Jul 6. J Biol Chem. 2018. PMID: 29980599 Free PMC article.
-
A full-length bifunctional protein involved in c-di-GMP turnover is required for long-term survival under nutrient starvation in Mycobacterium smegmatis.Microbiology (Reading). 2012 Jun;158(Pt 6):1415-1427. doi: 10.1099/mic.0.053892-0. Epub 2012 Feb 16. Microbiology (Reading). 2012. PMID: 22343354
-
[Activity of cyclic diguanylate (c-di-GMP) in bacteria and the study of its derivatives].Yao Xue Xue Bao. 2012 Mar;47(3):307-12. Yao Xue Xue Bao. 2012. PMID: 22645753 Review. Chinese.
-
Sensing the messenger: the diverse ways that bacteria signal through c-di-GMP.Protein Sci. 2012 Jul;21(7):929-48. doi: 10.1002/pro.2093. Epub 2012 Jun 5. Protein Sci. 2012. PMID: 22593024 Free PMC article. Review.
Cited by
-
Comparison of the transcriptome, lipidome, and c-di-GMP production between BCGΔBCG1419c and BCG, with Mincle- and Myd88-dependent induction of proinflammatory cytokines in murine macrophages.Sci Rep. 2024 May 24;14(1):11898. doi: 10.1038/s41598-024-61815-8. Sci Rep. 2024. PMID: 38789479 Free PMC article.
-
Diguanylate cyclase activity of the Mycobacterium leprae T cell antigen ML1419c.Microbiology (Reading). 2016 Sep;162(9):1651-1661. doi: 10.1099/mic.0.000339. Epub 2016 Jul 22. Microbiology (Reading). 2016. PMID: 27450520 Free PMC article.
-
Cyclic di-GMP: the first 25 years of a universal bacterial second messenger.Microbiol Mol Biol Rev. 2013 Mar;77(1):1-52. doi: 10.1128/MMBR.00043-12. Microbiol Mol Biol Rev. 2013. PMID: 23471616 Free PMC article. Review.
-
Regulation of the CRISPR-Associated Genes by Rv2837c (CnpB) via an Orn-Like Activity in Tuberculosis Complex Mycobacteria.J Bacteriol. 2018 Mar 26;200(8):e00743-17. doi: 10.1128/JB.00743-17. Print 2018 Apr 15. J Bacteriol. 2018. PMID: 29378893 Free PMC article.
-
Cyclic di-GMP regulates Mycobacterium tuberculosis resistance to ethionamide.Sci Rep. 2017 Jul 19;7(1):5860. doi: 10.1038/s41598-017-06289-7. Sci Rep. 2017. PMID: 28725053 Free PMC article.
References
-
- Miller MB, Bassler BL. Quorum sensing in bacteria. Ann Rev Microbiol. 2001;55:165–99. - PubMed
-
- Redfield JR. Is quorum sensing a side effect of diffusion sensing? Trends in Microbiol. 2002;10:365–70. - PubMed
-
- Jenal U. Cyclic di-guanosine-monophosphate comes of age: a novel secondary messenger involved in modulating cell surface structures in bacteria? Curr Opin Micorbiol. 2004:185–91. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

