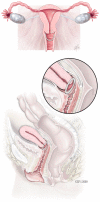
Examining the efficacy, safety, and patient acceptability of the combined contraceptive vaginal ring (NuvaRing)
- PMID: 21151688
- PMCID: PMC2990910
- DOI: 10.2147/IJWH.S6162
Examining the efficacy, safety, and patient acceptability of the combined contraceptive vaginal ring (NuvaRing)
Abstract
The contraceptive vaginal ring is a novel contraceptive method that offers unique advantages. Intravaginal delivery of both estrogen and progesterone allows continuous release of medication, resulting in lower systemic levels. The use of long-term combined hormonal contraception enables improved cycle control compared with progesterone-only methods. The indications and usage of the NuvaRing(®) are described along with the efficacy, tolerability, and safety. Overall, the contraceptive vaginal ring appears to be very effective, with a favorable side-effect profile, and is highly acceptable to most patients.
Keywords: NuvaRing; contraceptive agents; contraceptive device; ethinyl estradiol; etonogestrel; hormonal contraception; vaginal contraception; vaginal ring.
Figures
References
-
- Alexander NJ, Baker E, Miller L, Zampaglione E. Why consider vaginal drug administration? Fertil Steril. 2004;82:1–12. - PubMed
-
- Merki-Feld GS, Hund M. Clinical experience with NuvaRing in daily practice in Switzerland: cycle control and acceptability among women of all reproductive ages. Eur J Contracept Reprod Health Care. 2007;12:240–247. - PubMed
-
- Barnhart KT, Timbers K, Pretorius ES, Lin K, Shaunik A. In vivo assessment of NuvaRing placement. Contraception. 2005;72:196–199. - PubMed
-
- Roumen FJ, Apter D, Mulders TM, Dieben TO. Efficacy, tolerability and acceptability of a novel contraceptive vaginal ring releasing etonogestrel and ethinyl oestradiol. Hum Reprod. 2001;16:469–475. - PubMed
-
- Dieben TO, Roumen FJ, Apter D. Efficacy, cycle control, and user acceptability of a novel combined contraceptive vaginal ring. Obstet Gynecol. 2002;100:585–593. - PubMed
LinkOut - more resources
Full Text Sources