Rotavirus disrupts calcium homeostasis by NSP4 viroporin activity
- PMID: 21151776
- PMCID: PMC2999940
- DOI: 10.1128/mBio.00265-10
Rotavirus disrupts calcium homeostasis by NSP4 viroporin activity
Abstract
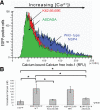
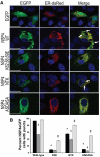
Many viruses alter intracellular calcium homeostasis. The rotavirus nonstructural protein 4 (NSP4), an endoplasmic reticulum (ER) transmembrane glycoprotein, increases intracellular levels of cytoplasmic Ca(2+) ([Ca(2+)]cyto) through a phospholipase C-independent pathway, which is required for virus replication and morphogenesis. However, the NSP4 domain and mechanism that increases [Ca(2+)]cyto are unknown. We identified an NSP4 domain (amino acids [aa] 47 to 90) that inserts into membranes and has structural characteristics of viroporins, a class of small hydrophobic viral proteins that disrupt membrane integrity and ion homeostasis to facilitate virus entry, assembly, or release. Mutational analysis showed that NSP4 viroporin activity was mediated by an amphipathic α-helical domain downstream of a conserved lysine cluster. The lysine cluster directed integral membrane insertion of the viroporin domain and was critical for viroporin activity. In epithelial cells, expression of wild-type NSP4 increased the levels of free cytoplasmic Ca(2+) by 3.7-fold, but NSP4 viroporin mutants maintained low levels of [Ca(2+)]cyto, were retained in the ER, and failed to form cytoplasmic vesicular structures, called puncta, which surround viral replication and assembly sites in rotavirus-infected cells. When [Ca(2+)]cyto was increased pharmacologically with thapsigargin, viroporin mutants formed puncta, showing that elevation of calcium levels and puncta formation are distinct functions of NSP4 and indicating that NSP4 directly or indirectly responds to elevated cytoplasmic calcium levels. NSP4 viroporin activity establishes the mechanism for NSP4-mediated elevation of [Ca(2+)]cyto, a critical event that regulates rotavirus replication and virion assembly.
Figures

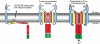
References
-
- Dubyak G. R. 2004. Ion homeostasis, channels, and transporters: an update on cellular mechanisms. Adv. Physiol. Educ. 28:143–154 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

