Dynamic vs static ABCG2 inhibitors to sensitize drug resistant cancer cells
- PMID: 21151870
- PMCID: PMC2998423
- DOI: 10.1371/journal.pone.0015276
Dynamic vs static ABCG2 inhibitors to sensitize drug resistant cancer cells
Abstract
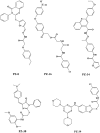
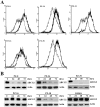
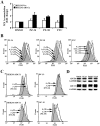
Human ABCG2, a member of the ATP-binding cassette transporter superfamily, plays a key role in multidrug resistance and protecting cancer stem cells. ABCG2-knockout had no apparent adverse effect on the development, biochemistry, and life of mice. Thus, ABCG2 is an interesting and promising target for development of chemo-sensitizing agents for better treatment of drug resistant cancers and for eliminating cancer stem cells. Previously, we reported a novel two mode-acting ABCG2 inhibitor, PZ-39, that induces ABCG2 degradation in addition to inhibiting its activity. In this manuscript, we report our recent progresses in identifying two different groups of ABCG2 inhibitors with one inhibiting only ABCG2 function (static) and the other induces ABCG2 degradation in lysosome in addition to inhibiting its function (dynamic). Thus, the inhibitor-induced ABCG2 degradation may be more common than we previously anticipated and further investigation of the dynamic inhibitors that induce ABCG2 degradation may provide a more effective way of sensitizing ABCG2-mediated MDR in cancer chemotherapy.
Conflict of interest statement
Figures
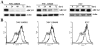



References
-
- Zhang JT. Biochemistry and pharmacology of the human multidrug resistance gene product, ABCG2. Zhong Nan Da Xue Xue Bao Yi Xue Ban (J Cent South Univ) 2007;32:531–541. - PubMed
-
- Xu J, Peng H, Zhang JT. Human multidrug transporter ABCG2, a target for sensitizing drug resistance in cancer chemotherapy. Curr Med Chem. 2007;14:689–701. - PubMed
-
- Xu J, Liu Y, Yang Y, Bates S, Zhang JT. Characterization of oligomeric human half-ABC transporter ATP-binding cassette G2. J Biol Chem. 2004;279:19781–19789. - PubMed
-
- McDevitt CA, Collins RF, Conway M, Modok S, Storm J, et al. Purification and 3D Structural Analysis of Oligomeric Human Multidrug Transporter ABCG2. Structure. 2006;14:1623–1632. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

