Intracellular trafficking of guanylate-binding proteins is regulated by heterodimerization in a hierarchical manner
- PMID: 21151871
- PMCID: PMC2998424
- DOI: 10.1371/journal.pone.0014246
Intracellular trafficking of guanylate-binding proteins is regulated by heterodimerization in a hierarchical manner
Abstract
Guanylate-binding proteins (GBPs) belong to the dynamin family of large GTPases and represent the major IFN-γ-induced proteins. Here we systematically investigated the mechanisms regulating the subcellular localization of GBPs. Three GBPs (GBP-1, GBP-2 and GBP-5) carry a C-terminal CaaX-prenylation signal, which is typical for small GTPases of the Ras family, and increases the membrane affinity of proteins. In this study, we demonstrated that GBP-1, GBP-2 and GBP-5 are prenylated in vivo and that prenylation is required for the membrane association of GBP-1, GBP-2 and GBP-5. Using co-immunoprecipitation, yeast-two-hybrid analysis and fluorescence complementation assays, we showed for the first time that GBPs are able to homodimerize in vivo and that the membrane association of GBPs is regulated by dimerization similarly to dynamin. Interestingly, GBPs could also heterodimerize. This resulted in hierarchical positioning effects on the intracellular localization of the proteins. Specifically, GBP-1 recruited GBP-5 and GBP-2 into its own cellular compartment and GBP-5 repositioned GBP-2. In addition, GBP-1, GBP-2 and GBP-5 were able to redirect non-prenylated GBPs to their compartment in a prenylation-dependent manner. Overall, these findings prove in vivo the ability of GBPs to dimerize, indicate that heterodimerization regulates sub-cellular localization of GBPs and underscore putative membrane-associated functions of this family of proteins.
Conflict of interest statement
Figures

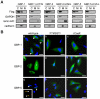
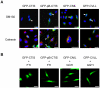
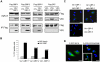
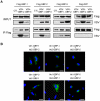

References
-
- Wennerberg K, Rossman KL, Der CJ. The Ras superfamily at a glance. J Cell Sci. 2005;118:843–846. - PubMed
-
- Vetter IR, Wittinghofer A. The guanine nucleotide-binding switch in three dimensions. Science. 2001;294:1299–1304. - PubMed
-
- Vogler O, Barcelo JM, Ribas C, Escriba PV. Membrane interactions of G proteins and other related proteins. Biochim Biophys Acta. 2008;1778:1640–1652. - PubMed
-
- Roskoski R., Jr Protein prenylation: a pivotal posttranslational process. Biochem Biophys Res Commun. 2003;303:1–7. - PubMed
-
- Rocks O, Peyker A, Kahms M, Verveer PJ, Koerner C, et al. An acylation cycle regulates localization and activity of palmitoylated Ras isoforms. Science. 2005;307:1746–1752. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

