Melanopsin contributions to irradiance coding in the thalamo-cortical visual system
- PMID: 21151887
- PMCID: PMC2998442
- DOI: 10.1371/journal.pbio.1000558
Melanopsin contributions to irradiance coding in the thalamo-cortical visual system
Abstract
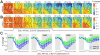
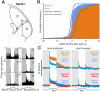
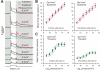
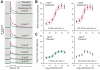
Photoreception in the mammalian retina is not restricted to rods and cones but extends to a subset of retinal ganglion cells expressing the photopigment melanopsin (mRGCs). These mRGCs are known to drive such reflex light responses as circadian photoentrainment and pupillomotor movements. By contrast, until now there has been no direct assessment of their contribution to conventional visual pathways. Here, we address this deficit. Using new reporter lines, we show that mRGC projections are much more extensive than previously thought and extend across the dorsal lateral geniculate nucleus (dLGN), origin of thalamo-cortical projection neurons. We continue to show that this input supports extensive physiological light responses in the dLGN and visual cortex in mice lacking rods+cones (a model of advanced retinal degeneration). Moreover, using chromatic stimuli to isolate melanopsin-derived responses in mice with an intact visual system, we reveal strong melanopsin input to the ∼40% of neurons in the LGN that show sustained activation to a light step. We demonstrate that this melanopsin input supports irradiance-dependent increases in the firing rate of these neurons. The implication that melanopsin is required to accurately encode stimulus irradiance is confirmed using melanopsin knockout mice. Our data establish melanopsin-based photoreception as a significant source of sensory input to the thalamo-cortical visual system, providing unique irradiance information and allowing visual responses to be retained even in the absence of rods+cones. These findings identify mRGCs as a potential origin for aspects of visual perception and indicate that they may support vision in people suffering retinal degeneration.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



Comment in
-
Melanopsin ganglion cells: a different way of seeing things.PLoS Biol. 2010 Dec 7;8(12):e1001003. doi: 10.1371/journal.pbio.1001003. PLoS Biol. 2010. PMID: 21151889 Free PMC article. Review. No abstract available.
References
-
- Belenky M. A, Smeraski C. A, Provencio I, Sollars P. J, Pickard G. E. Melanopsin retinal ganglion cells receive bipolar and amacrine cell synapses. J Comp Neurol. 2003;460:380–393. - PubMed
-
- Viney T. J, Balint K, Hillier D, Siegert S, Boldogkoi Z, et al. Local retinal circuits of melanopsin-containing ganglion cells identified by transsynaptic viral tracing. Curr Biol. 2007;17:981–988. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

