Atorvastatin improves survival in septic rats: effect on tissue inflammatory pathway and on insulin signaling
- PMID: 21151908
- PMCID: PMC2997789
- DOI: 10.1371/journal.pone.0014232
Atorvastatin improves survival in septic rats: effect on tissue inflammatory pathway and on insulin signaling
Erratum in
-
Correction: atorvastatin improves survival in septic rats: effect on tissue inflammatory pathway and on insulin signaling.PLoS One. 2015 Mar 3;10(3):e0118383. doi: 10.1371/journal.pone.0118383. eCollection 2015. PLoS One. 2015. PMID: 25734918 Free PMC article. No abstract available.
Retraction in
-
Retraction: Atorvastatin Improves Survival in Septic Rats: Effect on Tissue Inflammatory Pathway and on Insulin Signaling.PLoS One. 2016 Jul 8;11(7):e0159283. doi: 10.1371/journal.pone.0159283. eCollection 2016. PLoS One. 2016. PMID: 27391461 Free PMC article. No abstract available.
Abstract
The aim of the present study was to investigate whether the survival-improving effect of atorvastatin in sepsis is accompanied by a reduction in tissue activation of inflammatory pathways and, in parallel, an improvement in tissue insulin signaling in rats. Diffuse sepsis was induced by cecal ligation and puncture surgery (CLP) in male Wistar rats. Serum glucose and inflammatory cytokines levels were assessed 24 h after CLP. The effect of atorvastatin on survival of septic animals was investigated in parallel with insulin signaling and its modulators in liver, muscle and adipose tissue. Atorvastatin improves survival in septic rats and this improvement is accompanied by a marked improvement in insulin sensitivity, characterized by an increase in glucose disappearance rate during the insulin tolerance test. Sepsis induced an increase in the expression/activation of TLR4 and its downstream signaling JNK and IKK/NF-κB activation, and blunted insulin-induced insulin signaling in liver, muscle and adipose tissue; atorvastatin reversed all these alterations in parallel with a decrease in circulating levels of TNF-α and IL-6. In summary, this study demonstrates that atorvastatin treatment increased survival, with a significant effect upon insulin sensitivity, improving insulin signaling in peripheral tissues of rats during peritoneal-induced sepsis. The effect of atorvastatin on the suppression of the TLR-dependent inflammatory pathway may play a central role in regulation of insulin signaling and survival in sepsis insult.
Conflict of interest statement
Figures



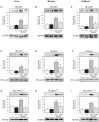

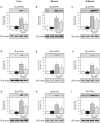
References
-
- Terblanche M, Almog Y, Rosenson RS, Smith TS, Hackam DG. Statins: panacea for sepsis? Lancet Infect Dis. 2006;6:242–248. - PubMed
-
- Almog Y. Statins, inflammation, and sepsis: hypothesis. Chest. 2003;124:740–743. - PubMed
-
- Maitra SR, Wojnar MM, Lang CH. Alterations in tissue glucose uptake during the hyperglycemic and hypoglycemic phases of sepsis. Shock. 2000;13:379–385. - PubMed
-
- Marik PE, Raghavan M. Stress-hyperglycemia, insulin and immunomodulation in sepsis. Intensive Care Med. 2004;30:748–756. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

