Short-term preservation of porcine oocytes in ambient temperature: novel approaches
- PMID: 21151922
- PMCID: PMC2998415
- DOI: 10.1371/journal.pone.0014242
Short-term preservation of porcine oocytes in ambient temperature: novel approaches
Abstract
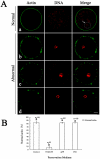
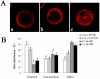
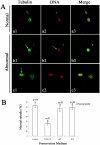
The objective of this study was to evaluate the feasibility of preserving porcine oocytes without freezing. To optimize preservation conditions, porcine cumulus-oocyte complexes (COCs) were preserved in TCM-199, porcine follicular fluid (pFF) and FCS at different temperatures (4°C, 20°C, 25°C, 27.5°C, 30°C and 38.5°C) for 1 day, 2 days or 3 days. After preservation, oocyte morphology, germinal vesicle (GV) rate, actin cytoskeleton organization, cortical granule distribution, mitochondrial translocation and intracellular glutathione level were evaluated. Oocyte maturation was indicated by first polar body emission and spindle morphology after in vitro culture. Strikingly, when COCs were stored at 27.5°C for 3 days in pFF or FCS, more than 60% oocytes were still arrested at the GV stage and more than 50% oocytes matured into MII stages after culture. Almost 80% oocytes showed normal actin organization and cortical granule relocation to the cortex, and approximately 50% oocytes showed diffused mitochondria distribution patterns and normal spindle configurations. While stored in TCM-199, all these criteria decreased significantly. Glutathione (GSH) level in the pFF or FCS group was higher than in the TCM-199 group, but lower than in the non-preserved control group. The preserved oocytes could be fertilized and developed to blastocysts (about 10%) with normal cell number, which is clear evidence for their retaining the developmental potentiality after 3d preservation. Thus, we have developed a simple method for preserving immature pig oocytes at an ambient temperature for several days without evident damage of cytoplasm and keeping oocyte developmental competence.
Conflict of interest statement
Figures


References
-
- Zhou GB, Li N. Cryopreservation of porcine oocytes: recent advances. Mol Hum Reprod. 2009;15:279–285. - PubMed
-
- Didion BA, Pomp D, Martin MJ, Homanics GE, Markert CL. Observations on the cooling and cryopreservation of pig oocytes at the germinal vesicle stage. J Anim Sci. 1990;68:2803–2810. - PubMed
-
- Nagashima H, Kashiwazaki N, Ashman RJ, Grupen CG, Seamark RF, et al. Removal of cytoplasmic lipid enhances the tolerance of porcine embryos to chilling. Biol Reprod. 1994;51:618–622. - PubMed
-
- Liu RH, Sun QY, Li YH, Jiao LH, Wang WH. Maturation of porcine oocytes after cooling at the germinal vesicle stage. Zygote. 2003;11:299–305. - PubMed
-
- Wu C, Rui R, Dai J, Zhang C, Ju S, et al. Effects of cryopreservation on the developmental competence, ultrastructure and cytoskeletal structure of porcine oocytes. Mol Reprod Dev. 2006;73:1454–1462. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

