Cationic amino acid transporters and Salmonella Typhimurium ArgT collectively regulate arginine availability towards intracellular Salmonella growth
- PMID: 21151933
- PMCID: PMC2997073
- DOI: 10.1371/journal.pone.0015466
Cationic amino acid transporters and Salmonella Typhimurium ArgT collectively regulate arginine availability towards intracellular Salmonella growth
Abstract
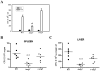
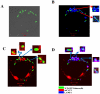
Cationic amino acid transporters (mCAT1 and mCAT2B) regulate the arginine availability in macrophages. How in the infected cell a pathogen can alter the arginine metabolism of the host remains to be understood. We reveal here a novel mechanism by which Salmonella exploit mCAT1 and mCAT2B to acquire host arginine towards its own intracellular growth within antigen presenting cells. We demonstrate that Salmonella infected bone marrow derived macrophages and dendritic cells show enhanced arginine uptake and increased expression of mCAT1 and mCAT2B. We show that the mCAT1 transporter is in close proximity to Salmonella containing vacuole (SCV) specifically by live intracellular Salmonella in order to access the macrophage cytosolic arginine pool. Further, Lysosome associated membrane protein 1, a marker of SCV, also was found to colocalize with mCAT1 in the Salmonella infected cell. The intra vacuolar Salmonella then acquire the host arginine via its own arginine transporter, ArgT for growth. The argT knockout strain was unable to acquire host arginine and was attenuated in growth in both macrophages and in mice model of infection. Together, these data reveal survival strategies by which virulent Salmonella adapt to the harsh conditions prevailing in the infected host cells.
Conflict of interest statement
Figures
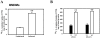



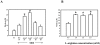


References
-
- Mori M, Gotoh T. Arginine metabolic enzymes, nitric oxide and infection. J Nutr. 2004;134:2820S–2825S; discussion 2853S. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

