Concerted regulation of cGMP and cAMP phosphodiesterases in early cardiac hypertrophy induced by angiotensin II
- PMID: 21151982
- PMCID: PMC2997062
- DOI: 10.1371/journal.pone.0014227
Concerted regulation of cGMP and cAMP phosphodiesterases in early cardiac hypertrophy induced by angiotensin II
Abstract
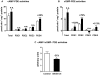
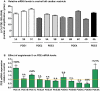
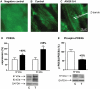
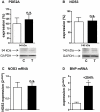
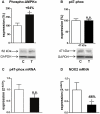
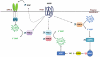
Left ventricular hypertrophy leads to heart failure and represents a high risk leading to premature death. Cyclic nucleotides (cAMP and cGMP) play a major role in heart contractility and cyclic nucleotide phosphodiesterases (PDEs) are involved in different stages of advanced cardiac diseases. We have investigated their contributions in the very initial stages of left ventricular hypertrophy development. Wistar male rats were treated over two weeks by chronic infusion of angiotensin II using osmotic mini-pumps. Left cardiac ventricles were used as total homogenates for analysis. PDE1 to PDE5 specific activities and protein and mRNA expressions were explored.Rats developed arterial hypertension associated with a slight cardiac hypertrophy (+24%). cAMP-PDE4 activity was specifically increased while cGMP-PDE activities were broadly increased (+130% for PDE1; +76% for PDE2; +113% for PDE5) and associated with increased expressions for PDE1A, PDE1C and PDE5A. The cGMP-PDE1 activation by Ca(2+)/CaM was reduced. BNP expression was increased by 3.5-fold, while NOX2 expression was reduced by 66% and AMP kinase activation was increased by 64%. In early cardiac hypertrophy induced by angiotensin II, all specific PDE activities in left cardiac ventricles were increased, favoring an increase in cGMP hydrolysis by PDE1, PDE2 and PDE5. Increased cAMP hydrolysis was related to PDE4. We observed the establishment of two cardioprotective mechanisms and we suggest that these mechanisms could lead to increase intracellular cGMP: i) increased expression of BNP could increase "particulate" cGMP pool; ii) increased activation of AMPK, subsequent to increase in PDE4 activity and 5'AMP generation, could elevate "soluble" cGMP pool by enhancing NO bioavailability through NOX2 down-regulation. More studies are needed to support these assumptions. Nevertheless, our results suggest a potential link between PDE4 and AMPK/NOX2 and they point out that cGMP-PDEs, especially PDE1 and PDE2, may be interesting therapeutic targets in preventing cardiac hypertrophy.
Conflict of interest statement
Figures

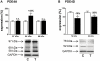

References
-
- Bayes-Genis A, Guindo J, Vinolas X, Tomas L, Elosua R, et al. Cardiac arrhythmias and left ventricular hypertrophy in systemic hypertension and their influences on prognosis. Am J Cardiol. 1995;76:54D–59D. - PubMed
-
- Weber KT, Brilla CG. Structural basis for pathologic left ventricular hypertrophy. Clin Cardiol. 1993;16:II10–14. - PubMed
-
- Serneri GG, Boddi M, Cecioni I, Vanni S, Coppo M, et al. Cardiac angiotensin II formation in the clinical course of heart failure and its relationship with left ventricular function. Circ Res. 2001;88:961–968. - PubMed
-
- Mehta PK, Griendling KK. Angiotensin II cell signaling: physiological and pathological effects in the cardiovascular system. Am J Physiol Cell Physiol. 2007;292:C82–97. - PubMed
-
- Touyz RM, Schiffrin EL. Signal transduction mechanisms mediating the physiological and pathophysiological actions of angiotensin II in vascular smooth muscle cells. Pharmacol Rev. 2000;52:639–672. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

