A systems model for immune cell interactions unravels the mechanism of inflammation in human skin
- PMID: 21152006
- PMCID: PMC2996319
- DOI: 10.1371/journal.pcbi.1001024
A systems model for immune cell interactions unravels the mechanism of inflammation in human skin
Abstract
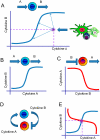
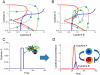
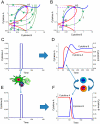
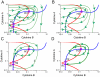
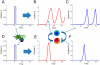
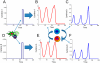
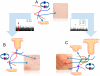
Inflammation is characterized by altered cytokine levels produced by cell populations in a highly interdependent manner. To elucidate the mechanism of an inflammatory reaction, we have developed a mathematical model for immune cell interactions via the specific, dose-dependent cytokine production rates of cell populations. The model describes the criteria required for normal and pathological immune system responses and suggests that alterations in the cytokine production rates can lead to various stable levels which manifest themselves in different disease phenotypes. The model predicts that pairs of interacting immune cell populations can maintain homeostatic and elevated extracellular cytokine concentration levels, enabling them to operate as an immune system switch. The concept described here is developed in the context of psoriasis, an immune-mediated disease, but it can also offer mechanistic insights into other inflammatory pathologies as it explains how interactions between immune cell populations can lead to disease phenotypes.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




Similar articles
-
Tyk2 is a therapeutic target for psoriasis-like skin inflammation.Int Immunol. 2014 May;26(5):257-67. doi: 10.1093/intimm/dxt062. Epub 2013 Dec 17. Int Immunol. 2014. PMID: 24345760
-
Calcipotriol and betamethasone dipropionate exert additive inhibitory effects on the cytokine expression of inflammatory dendritic cell-Th17 cell axis in psoriasis.J Dermatol Sci. 2016 Mar;81(3):153-64. doi: 10.1016/j.jdermsci.2015.12.009. Epub 2015 Dec 23. J Dermatol Sci. 2016. PMID: 26794805
-
Gene from a psoriasis susceptibility locus primes the skin for inflammation.Sci Transl Med. 2010 Dec 8;2(61):61ra90. doi: 10.1126/scitranslmed.3001108. Sci Transl Med. 2010. PMID: 21148126 Free PMC article.
-
Regulation of cytokines by small RNAs during skin inflammation.J Biomed Sci. 2010 Jul 1;17(1):53. doi: 10.1186/1423-0127-17-53. J Biomed Sci. 2010. PMID: 20594301 Free PMC article. Review.
-
Innate immunity in the pathogenesis of psoriasis.Arch Dermatol Res. 2011 Dec;303(10):691-705. doi: 10.1007/s00403-011-1169-1. Epub 2011 Aug 24. Arch Dermatol Res. 2011. PMID: 21863252 Review.
Cited by
-
Serum and cerebrospinal fluid immune mediators in children with autistic disorder: a longitudinal study.Mol Autism. 2017 Jan 5;8:1. doi: 10.1186/s13229-016-0115-7. eCollection 2017. Mol Autism. 2017. PMID: 28070266 Free PMC article. Clinical Trial.
-
Transcriptome classification reveals molecular subtypes in psoriasis.BMC Genomics. 2012 Sep 12;13:472. doi: 10.1186/1471-2164-13-472. BMC Genomics. 2012. PMID: 22971201 Free PMC article.
-
Systems approaches to computational modeling of the oral microbiome.Front Physiol. 2013 Jul 10;4:172. doi: 10.3389/fphys.2013.00172. eCollection 2013. Front Physiol. 2013. PMID: 23847548 Free PMC article.
-
Immunology: A many layered thing.Nature. 2012 Dec 20;492(7429):S52-4. doi: 10.1038/492S52a. Nature. 2012. PMID: 23254971 No abstract available.
-
The effect of muscle length on post-tetanic potentiation of C57BL/6 and skMLCK-/- mouse EDL muscles.J Muscle Res Cell Motil. 2022 Sep;43(3):99-111. doi: 10.1007/s10974-022-09620-6. Epub 2022 Jun 30. J Muscle Res Cell Motil. 2022. PMID: 35771335
References
-
- Yarilin AA, Belyakov IM. Cytokines in the thymus: production and biological effects. Curr Med Chem. 2004;11:447–464. - PubMed
-
- Nickoloff BJ, Xin H, Nestle FO, Qin JZ. The cytokine and chemokine network in psoriasis. Clin Dermatol. 2007;25:568–573. - PubMed
-
- Cutolo M, Bijlsma JW, Lahita RG, Masi AT, Straub RH, et al. Altered neuroendocrine immune (NEI) networks in rheumatology. Ann N Y Acad Sci. 2002;966:xiii–xviii. - PubMed
-
- Pinilla-Ibarz J, Shah B, Dubovsky JA. The biological basis for immunotherapy in patients with chronic myelogenous leukemia. Cancer Control. 2009;16:141–152. - PubMed
-
- Erickson B, Sperber K, Frishman WH. Toll-like receptors: new therapeutic targets for the treatment of atherosclerosis, acute coronary syndromes, and myocardial failure. Cardiol Rev. 2008;16:273–279. - PubMed

