Self-organized criticality in developing neuronal networks
- PMID: 21152008
- PMCID: PMC2996321
- DOI: 10.1371/journal.pcbi.1001013
Self-organized criticality in developing neuronal networks
Abstract
Recently evidence has accumulated that many neural networks exhibit self-organized criticality. In this state, activity is similar across temporal scales and this is beneficial with respect to information flow. If subcritical, activity can die out, if supercritical epileptiform patterns may occur. Little is known about how developing networks will reach and stabilize criticality. Here we monitor the development between 13 and 95 days in vitro (DIV) of cortical cell cultures (n = 20) and find four different phases, related to their morphological maturation: An initial low-activity state (≈19 DIV) is followed by a supercritical (≈20 DIV) and then a subcritical one (≈36 DIV) until the network finally reaches stable criticality (≈58 DIV). Using network modeling and mathematical analysis we describe the dynamics of the emergent connectivity in such developing systems. Based on physiological observations, the synaptic development in the model is determined by the drive of the neurons to adjust their connectivity for reaching on average firing rate homeostasis. We predict a specific time course for the maturation of inhibition, with strong onset and delayed pruning, and that total synaptic connectivity should be strongly linked to the relative levels of excitation and inhibition. These results demonstrate that the interplay between activity and connectivity guides developing networks into criticality suggesting that this may be a generic and stable state of many networks in vivo and in vitro.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


 (
( indicates the standard deviation), which are given in the inset Table, of the associated state.
indicates the standard deviation), which are given in the inset Table, of the associated state.  amount of action potentials per minute, therefore, mean activity.
amount of action potentials per minute, therefore, mean activity.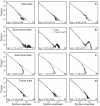

 , calcium concentration
, calcium concentration  , axonal supply
, axonal supply  and dendritic acceptance
and dendritic acceptance  , and connectivity
, and connectivity  between dendrite
between dendrite  and axon
and axon  and the constant homeostatic value
and the constant homeostatic value  . The up and down arrows indicate if a variable is increased/decreased. For details see main text. (B) The mean synaptic density
. The up and down arrows indicate if a variable is increased/decreased. For details see main text. (B) The mean synaptic density  develops comparable to experimental findings in cell cultures (see inset from [24]). Note, time axis has been stretched in the middle. (C) Development of the average axonal supplies and dendritic acceptances. The network model passes through three different developmental phases: the first phase is characterized by a pronounced increase of the dendritic acceptance. During development, the network undergoes a transition (second phase) and finally it reaches a homeostatic equilibrium (third phase) with more axonal supplies than dendritic acceptances. (D) Network activity and calcium concentration change accordingly. At the beginning, activity rises slowly until a transition happens. During the transition, activity reaches its maximum and subsequently decreases to a homeostatic value.
develops comparable to experimental findings in cell cultures (see inset from [24]). Note, time axis has been stretched in the middle. (C) Development of the average axonal supplies and dendritic acceptances. The network model passes through three different developmental phases: the first phase is characterized by a pronounced increase of the dendritic acceptance. During development, the network undergoes a transition (second phase) and finally it reaches a homeostatic equilibrium (third phase) with more axonal supplies than dendritic acceptances. (D) Network activity and calcium concentration change accordingly. At the beginning, activity rises slowly until a transition happens. During the transition, activity reaches its maximum and subsequently decreases to a homeostatic value.
 (B:
(B:  ; C:
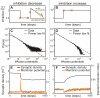
; C:  ), the avalanche distribution turns from a Poisson into a power-law like distribution similar to Figure 3 A. (bottom): In Phase II without inhibition (D), no real avalanche distribution can be observed and one sees only one or two “avalanches” (marked by a cross). Adding inhibition brings the system back into a stable, albeit supercritical regime. Within a wide tested range (Table 2), the amount of inhibition does not significantly change the degree of supercriticality. (E) Network with weak inhibition
), the avalanche distribution turns from a Poisson into a power-law like distribution similar to Figure 3 A. (bottom): In Phase II without inhibition (D), no real avalanche distribution can be observed and one sees only one or two “avalanches” (marked by a cross). Adding inhibition brings the system back into a stable, albeit supercritical regime. Within a wide tested range (Table 2), the amount of inhibition does not significantly change the degree of supercriticality. (E) Network with weak inhibition  and (F) with strong inhibition
and (F) with strong inhibition  .
.
 ). (B) If the absolute value of the inhibitory strength
). (B) If the absolute value of the inhibitory strength  equals the excitatory strength
equals the excitatory strength  the network becomes critical (
the network becomes critical ( ). Here the total number of inhibitory synapses is about 20%. (C–D) Higher levels of inhibition (
). Here the total number of inhibitory synapses is about 20%. (C–D) Higher levels of inhibition ( for C and
for C and  for D) keep the network in a subcritical regime (C:
for D) keep the network in a subcritical regime (C:  ; D:
; D:  ).
).
 or (D) subcritical (
or (D) subcritical ( ), respectively. Distributions were plotted at the time point marked by the open disks in A and B. The reduced inhibition case is well backed-up by experimental data as a similar change in criticality was observed in mature cell cultures after artificially increasing inhibition (compare inset). After some time (open square marker) distributions change and are then those shown in panels A and D in Figure 6. Now we have in both cases somewhat reduced (absolute)
), respectively. Distributions were plotted at the time point marked by the open disks in A and B. The reduced inhibition case is well backed-up by experimental data as a similar change in criticality was observed in mature cell cultures after artificially increasing inhibition (compare inset). After some time (open square marker) distributions change and are then those shown in panels A and D in Figure 6. Now we have in both cases somewhat reduced (absolute)  values as compared to those directly after the jump (now
values as compared to those directly after the jump (now  for the supercritical case Figure 6 A and
for the supercritical case Figure 6 A and  for the subcritical case Figure 6 D). Note, however, that we do not get back to the initial criticality (Figure 6 B,
for the subcritical case Figure 6 D). Note, however, that we do not get back to the initial criticality (Figure 6 B,  ). Parallel to this, the bottom panels (E,F) show that in both cases connectivity remains also changed. Activity, on the other hand, fully builds back.
). Parallel to this, the bottom panels (E,F) show that in both cases connectivity remains also changed. Activity, on the other hand, fully builds back.
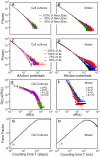
 against the mean connectivity
against the mean connectivity  described by Equation 10 is displayed together with its possible trajectories (blue).
described by Equation 10 is displayed together with its possible trajectories (blue).  marks the equilibrium or stable point of the network. (B) Hysteresis curve from the simulation. (C) Different representation, which shows that the equilibrium
marks the equilibrium or stable point of the network. (B) Hysteresis curve from the simulation. (C) Different representation, which shows that the equilibrium  represents a region of fixed points with approximately equal connectivity. The axes represent here axonal supply and dendritic acceptance. Color indicates the calculated average connectivity
represents a region of fixed points with approximately equal connectivity. The axes represent here axonal supply and dendritic acceptance. Color indicates the calculated average connectivity  . Depending on the initial state, the model grows into a fixed point of an omega limit set (yellow circles, region
. Depending on the initial state, the model grows into a fixed point of an omega limit set (yellow circles, region  ) lying on a hyperbola (dashed line), thus with approximately equal connectivity
) lying on a hyperbola (dashed line), thus with approximately equal connectivity  . The “bumpy” shape of the hyperbola is due to grid aliasing effects.
. The “bumpy” shape of the hyperbola is due to grid aliasing effects.
 and, therefore, the scale-free behavior of model and cell cultures. Panels (G,H) show a Fano factor analysis for cell culture and model in the critical state. The exponent of the Fano Factor (linear regression) is
and, therefore, the scale-free behavior of model and cell cultures. Panels (G,H) show a Fano factor analysis for cell culture and model in the critical state. The exponent of the Fano Factor (linear regression) is  for the model and
for the model and  for cell cultures. Hence we conclude a scale-free clustering over different time scales
for cell cultures. Hence we conclude a scale-free clustering over different time scales  .
.Similar articles
-
Structural Modularity Tunes Mesoscale Criticality in Biological Neuronal Networks.J Neurosci. 2023 Apr 5;43(14):2515-2526. doi: 10.1523/JNEUROSCI.1420-22.2023. Epub 2023 Mar 3. J Neurosci. 2023. PMID: 36868860 Free PMC article.
-
Neurobiologically realistic determinants of self-organized criticality in networks of spiking neurons.PLoS Comput Biol. 2011 Jun;7(6):e1002038. doi: 10.1371/journal.pcbi.1002038. Epub 2011 Jun 2. PLoS Comput Biol. 2011. PMID: 21673863 Free PMC article.
-
Temporal relation between neural activity and neurite pruning on a numerical model and a microchannel device with micro electrode array.Biochem Biophys Res Commun. 2017 Apr 29;486(2):539-544. doi: 10.1016/j.bbrc.2017.03.082. Epub 2017 Mar 18. Biochem Biophys Res Commun. 2017. PMID: 28322793
-
Propagation delays determine neuronal activity and synaptic connectivity patterns emerging in plastic neuronal networks.Chaos. 2018 Oct;28(10):106308. doi: 10.1063/1.5037309. Chaos. 2018. PMID: 30384625 Review.
-
The criticality hypothesis: how local cortical networks might optimize information processing.Philos Trans A Math Phys Eng Sci. 2008 Feb 13;366(1864):329-43. doi: 10.1098/rsta.2007.2092. Philos Trans A Math Phys Eng Sci. 2008. PMID: 17673410 Review.
Cited by
-
Automated quantification of neuronal networks and single-cell calcium dynamics using calcium imaging.J Neurosci Methods. 2015 Mar 30;243:26-38. doi: 10.1016/j.jneumeth.2015.01.020. Epub 2015 Jan 25. J Neurosci Methods. 2015. PMID: 25629800 Free PMC article.
-
Homeostatic plasticity and emergence of functional networks in a whole-brain model at criticality.Sci Rep. 2018 Oct 24;8(1):15682. doi: 10.1038/s41598-018-33923-9. Sci Rep. 2018. PMID: 30356174 Free PMC article.
-
Network bursting dynamics in excitatory cortical neuron cultures results from the combination of different adaptive mechanisms.PLoS One. 2013 Oct 11;8(10):e75824. doi: 10.1371/journal.pone.0075824. eCollection 2013. PLoS One. 2013. PMID: 24146781 Free PMC article.
-
How Memory Conforms to Brain Development.Front Comput Neurosci. 2019 Apr 16;13:22. doi: 10.3389/fncom.2019.00022. eCollection 2019. Front Comput Neurosci. 2019. PMID: 31057385 Free PMC article.
-
Criticality, Connectivity, and Neural Disorder: A Multifaceted Approach to Neural Computation.Front Comput Neurosci. 2021 Feb 10;15:611183. doi: 10.3389/fncom.2021.611183. eCollection 2021. Front Comput Neurosci. 2021. PMID: 33643017 Free PMC article. Review.
References
-
- Chialvo D. Critical brain networks. Physica A. 2004;340:756–765.
-
- Plenz D, Thiagarajan T. The organizing principles of neuronal avalanches: Cell assemblies in the cortex. Trends Neurosci. 2007;30:101–110. - PubMed
-
- Gutenberg B, Richter C. Seismicity of the earth. Princeton: Princeton University Press; 1956. 273
-
- Harris T. The theory of branching processes. New York: Dover Publications; 1989. 230
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

