In vitro and in vivo anti-inflammatory activity of 17-O-acetylacuminolide through the inhibition of cytokines, NF-κB translocation and IKKβ activity
- PMID: 21152019
- PMCID: PMC2995738
- DOI: 10.1371/journal.pone.0015105
In vitro and in vivo anti-inflammatory activity of 17-O-acetylacuminolide through the inhibition of cytokines, NF-κB translocation and IKKβ activity
Abstract
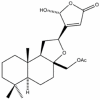
Background and purpose: 17-O-acetylacuminolide (AA), a diterpenoid labdane, was isolated for the first time from the plant species Neouvaria foetida. The anti-inflammatory effects of this compound were studied both in vitro and in vivo.
Experimental approach: Plant extracts were initially tested against LPS-stimulated release of tumor necrosis factor alpha (TNF-α) from murine macrophages (RAW264.7 cells). Based on bioassay-guided fractionation, the active compound was identified as AA. AA was tested for its ability to reduce nitric oxide (NO) production, and the inducible nitric oxide synthase (iNOS) expression. The inhibition of a panel of inflammatory cytokines (TNF, IL-1β, IL-6, KC, and GM-CSF) by AA was assessed at the expression and the mRNA levels. Moreover, the effect of AA on the translocation of the transcription factor nuclear factor kappa B (NF-κB) was evaluated in LPS-stimulated RAW264.7 cells and in TNF-stimulated L929 cells. Subsequently, AA was tested in the inhibitor of NF-κB kinase beta (IKKβ) activity assay. Lastly, the anti-inflammatory activity of AA in vivo was evaluated by testing TNF production in LPS-stimulated Balb/c mice.
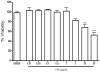
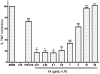
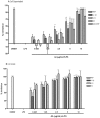
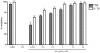
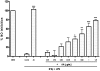
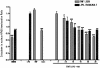
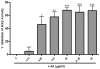
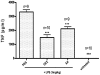
Key results: AA effectively inhibited TNF-α release with an IC(50) of 2.7 µg/mL. Moreover, AA significantly inhibited both NO production and iNOS expression. It significantly and dose-dependently inhibited TNF and IL-1β proteins and mRNA expression; as well as IL-6 and KC proteins. Additionally, AA prevented the translocation of NF-κB in both cell lines; suggesting that it is acting at a post receptor level. This was confirmed by AA's ability to inhibit IKKβ activity, a kinase responsible for activating NF-κB, hence providing an insight on AA's mechanism of action. Finally, AA significantly reduced TNF production in vivo.
Conclusions and implications: This study presents the potential utilization of this compound, as a lead for the development of an anti-inflammatory drug.
Conflict of interest statement
Figures




References
-
- Medzhitov R. Origin and physiological roles of inflammation. Nature. 2008;454:428–435. - PubMed
-
- Serhan CN. Resolution Phase of Inflammation: Novel Endogenous Anti-Inflammatory and Proresolving Lipid Mediators and Pathways. Annu Rev Immunol. 2007;25:101–137. - PubMed
-
- Schett G. Rheumatoid arthritis: inflammation and bone loss. Wien Med Wochenschr. 2006;156:34–41. - PubMed
-
- Karin M, Greten FR. NF-kappaB: linking inflammation and immunity to cancer development and progression. Nat Rev Immunol. 2005;5:749–759. - PubMed
-
- Libby P, Ridker P, Maseri A. Inflammation and Atherosclerosis. Circulation. 2002;105:1135–1143. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

