A full pharmacological analysis of the three turkey β-adrenoceptors and comparison with the human β-adrenoceptors
- PMID: 21152092
- PMCID: PMC2994877
- DOI: 10.1371/journal.pone.0015487
A full pharmacological analysis of the three turkey β-adrenoceptors and comparison with the human β-adrenoceptors
Abstract
Background: There are three turkey β-adrenoceptors: the original turkey β-adrenoceptor from erythrocytes (tβtrunc, for which the X-ray crystal structure has recently been determined), tβ3C and tβ4C-receptors. This study examined the similarities and differences between these avian receptors and mammalian receptors with regards to binding characteristics and functional high and low affinity agonist conformations.
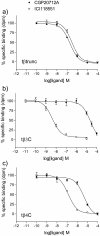
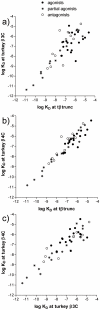
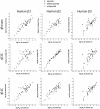
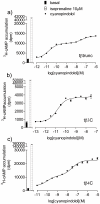
Methodology/principal findings: Stable cell lines were constructed with each of the turkey β-adrenoceptors and 3H-CGP12177 whole cell binding, CRE-SPAP production and (3)H-cAMP accumulation assays performed. It was confirmed that the three turkey β-adrenoceptors are distinct from each other in terms of amino acid sequence and binding characteristics. The greatest similarity of any of the turkey β-adrenoceptors to human β-adrenoceptors is between the turkey β3C-receptor and the human β2-adrenoceptor. There are pharmacologically distinct differences between the binding of ligands for the tβtrunc and tβ4C and the human β-adrenoceptors (e.g. with CGP20712A and ICI118551). The tβtrunc and tβ4C-adrenoceptors appear to exist in at least two different agonist conformations in a similar manner to that seen at both the human and rat β1-adrenoceptor and human β3-adrenoceptors. The tβ3C-receptor, similar to the human β2-adrenoceptor, does not, at least so far, appear to exist in more than one agonist conformation.
Conclusions/significance: There are several similarities, but also several important differences, between the recently crystallised turkey β-adrenoceptor and the human β-adrenoceptors. These findings are important for those the field of drug discovery using the recently structural information from crystallised receptors to aid drug design. Furthermore, comparison of the amino-acid sequence for the turkey and human adrenoceptors may therefore shed more light on the residues involved in the existence of the secondary β-adrenoceptor conformation.
Conflict of interest statement
Figures





References
-
- Pak MD, Fishman PH. Anomalous behaviour of CGP 12177A on beta 1-adrenergic receptors. J Recept Signal Transduction Res. 1996;16:1–23. - PubMed
-
- Granneman JG. The putative beta4-adrenergic receptor is a novel state of the beta1-adrenergic receptor. Am J Physiol Endocrinol Metab. 2001;280:E199–202. - PubMed
-
- Kaumann AJ, Molenaar P. The low-affinity site of the beta1-adrenoceptor and its relevance to cardiovascular pharmacology. Pharmacol Ther. 2008;118:303–336. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

